Chonnam Med J.
2018 Jan;54(1):55-62. 10.4068/cmj.2018.54.1.55.
Safety and Efficacy of the Endeavor Resolute® Stent in Patients with Multivessel Disease: The HEART (Honam EndeAvor ResoluTe) Prospective, Multicenter Trial
- Affiliations
-
- 1Department of Cardiovascular Medicine, Chonnam National University Hospital, Gwangju, Korea. myungho@chollian.net
- 2Department of Cardiology, Chosun University Hospital, Gwangju, Korea.
- 3Department of Cardiology, Kwangju Veterans Hospital, Gwangju, Korea.
- 4Department of Cardiology, Kwangju Christian Hospital, Gwangju, Korea.
- 5Department of Cardiology, Mokpo Jungang Hospital, Mokpo, Korea.
- 6Department of Cardiology, Jeonju Presbyterian Medical Center, Jeonju, Korea.
- 7Department of Cardiology, Chunbuk National University Hospital, Jeonju, Korea.
- 8Department of Cardiology, Wonkwang University Hospital, Iksan, Korea.
- 9Department of Cardiology, St. Carollo Hospital, Suncheon, Korea.
- KMID: 2458586
- DOI: http://doi.org/10.4068/cmj.2018.54.1.55
Abstract
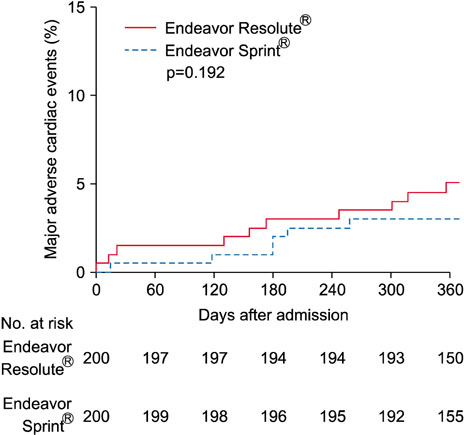
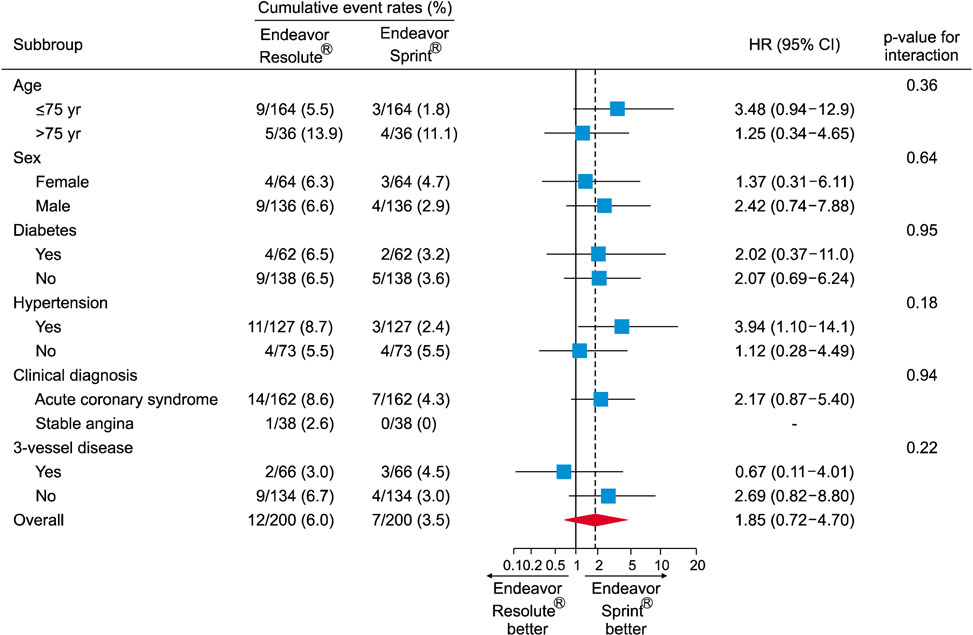
- The Endeavor Resolute® (ER) is a zotarolimus-eluting stent (ZES) with a biocompatible BioLinx polymer. This study prospectively compared the clinical outcomes of 2 versions of ZES, ER and Endeavor Sprint® (ES), in patients with multivessel disease. A total of 488 patients who underwent multivessel percutaneous coronary intervention (PCI) were divided into 2 groups the ER group (n=288) and the ES group (n=200). The primary endpoint was a composite of major adverse cardiac events (MACE) consisting of death, myocardial infarction, and target vessel revascularization after 12 months. In all patients, the prevalence of diabetes was higher in the ER group (42.7% vs. 31.0%, p=0.009). The rate of post-PCI Thrombolysis in Myocardial Infarction flow grade 3 was higher in the ER group (100.0% vs. 98.0%, p=0.028). There were no between-group differences in the in-hospital, 1-month and 12-month clinical outcomes. In the propensity score matched cohort (n=200 in each group), no differences were observed in the baseline and procedural characteristics. There were no statistical differences in the rates of in-hospital, 1-month and 12-month events (12-month MACE in the ER and ES groups: 6.0% vs. 3.5%, p=0.240, respectively). The safety and efficacy of both versions of ZES were comparable in patients with multivessel disease during a 12-month clinical follow-up.
MeSH Terms
Figure
Reference
-
1. Kandzari DE, Leon MB. Overview of pharmacology and clinical trials program with the zotarolimus-eluting endeavor stent. J Interv Cardiol. 2006; 19:405–413.
Article2. Meredith IT, Worthley S, Whitbourn R, Walters DL, McClean D, Horrigan M, et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc Interv. 2009; 2:977–985.
Article3. Williams PD, Awan M. Stent selection for percutaneous coronary intervention. Cont Cardiol Educ. 2017; 3:64–69.
Article4. Price MJ, Shlofmitz RA, Spriggs DJ, Haldis TA, Myers P, Popma Almonacid A, et al. Safety and efficacy of the next generation Resolute Onyx zotarolimus-eluting stent: primary outcome of the RESOLUTE ONYX core trial. Catheter Cardiovasc Interv. 2017; DOI: 10.1002/ccd.27322. [Epub ahead of print].
Article5. Kandzari DE, Leon MB, Popma JJ, Fitzgerald PJ, O'Shaughnessy C, Ball MW, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol. 2006; 48:2440–2447.
Article6. Eisenstein EL, Leon MB, Kandzari DE, Mauri L, Edwards R, Kong DF, et al. Long-term clinical and economic analysis of the Endeavor zotarolimus-eluting stent versus the cypher sirolimus-eluting stent: 3-year results from the ENDEAVOR III trial (Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv. 2009; 2:1199–1207.
Article7. Rasmussen K, Maeng M, Kaltoft A, Thayssen P, Kelbaek H, Tilsted HH, et al. Efficacy and safety of zotarolimus-eluting and sirolimus-eluting coronary stents in routine clinical care (SORT OUT III): a randomised controlled superiority trial. Lancet. 2010; 375:1090–1099.
Article8. Maeng M, Tilsted HH, Jensen LO, Kaltoft A, Kelbæk H, Abildgaard U, et al. 3-Year clinical outcomes in the randomized SORT OUT III superiority trial comparing zotarolimus- and sirolimus-eluting coronary stents. JACC Cardiovasc Interv. 2012; 5:812–818.
Article9. Maeng M, Tilsted HH, Jensen LO, Krusell LR, Kaltoft A, Kelbæk H, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014; 383:2047–2056.
Article10. Leon MB, Mauri L, Popma JJ, Cutlip DE, Nikolsky E, O'Shaughnessy C, et al. A randomized comparison of the Endeavor zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J Am Coll Cardiol. 2010; 55:543–554.
Article11. Kirtane AJ, Leon MB, Ball MW, Bajwa HS, Sketch MH Jr, Coleman PS, et al. The “final” 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovasc Interv. 2013; 6:325–333.
Article12. Park DW, Kim YH, Yun SC, Kang SJ, Lee SW, Lee CW, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. J Am Coll Cardiol. 2010; 56:1187–1195.13. Tada T, Byrne RA, Cassese S, King L, Schulz S, Mehilli J, et al. Comparative efficacy of 2 zotarolimus-eluting stent generations: resolute versus endeavor stents in patients with coronary artery disease. Am Heart J. 2013; 165:80–86.
Article14. Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007; 356:1020–1029.
Article15. D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998; 17:2265–2281.16. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999; 150:327–333.
Article17. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985; 39:33–38.
Article18. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011; 10:150–161.
Article19. Cohen J. The t test for means. In : Cohen J, editor. Statistical poweranalysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates;1988. p. 19–74.20. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001; 54:387–398.
Article21. Atalar E, Haznedaroğlu I, Aytemir K, Aksöyek S, Ovünç K, Oto A, et al. Effects of stent coating on platelets and endothelial cells after intracoronary stent implantation. Clin Cardiol. 2001; 24:159–164.
Article22. Malik N, Gunn J, Shepherd L, Crossman DC, Cumberland DC, Holt CM. Phosphorylcholine-coated stents in porcine coronary arteries: in vivo assessment of biocompatibility. J Invasive Cardiol. 2001; 13:193–201.23. Lewis AL, Tolhurst LA, Stratford PW. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre- and post-implantation. Biomaterials. 2002; 23:1697–1706.
Article24. Udipi K, Melder RJ, Chen M, Cheng P, Hezi-Yamit A, Sullivan C, et al. The next generation Endeavor Resolute Stent: role of the BioLinx Polymer System. EuroIntervention. 2007; 3:137–139.25. Udipi K, Chen M, Cheng P, Jiang K, Judd D, Caceres A, et al. Development of a novel biocompatible polymer system for extended drug release in a next-generation drug-eluting stent. J Biomed Mater Res A. 2008; 85:1064–1071.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osstem Cardiotec Centum Stent Versus Xience Alpine Stent for De Novo Coronary Artery Lesion: A Multicenter, Randomized, Parallel-Designed, Single Blind Test
- Simultaneous Multi-Vessel Subacute Stent Thromboses in Zotarolimus-Eluting Stents
- Current Status of Coronary Intervention in Patients with ST-Segment Elevation Myocardial Infarction and Multivessel Coronary Artery Disease
- A Prospective Randomized Trial of Corticosteroids for the Prevention of Restenosis after Intracoronary Stent Implantation
- A First-in-Man Clinical Evaluation of Sirolimus and Ascorbic Acid-Eluting Stent Systems: a Multicenter, Subject-Blinded, Randomized Study