Korean J Physiol Pharmacol.
2019 Sep;23(5):295-303. 10.4196/kjpp.2019.23.5.295.
Clinical and pharmacological application of multiscale multiphysics heart simulator, UT-Heart
- Affiliations
-
- 1UT-Heart Inc., Tokyo 154-0003, Japan. okada@sml.k.u-tokyo.ac.jp
- 2Future Center Initiative, The University of Tokyo, Chiba 277-0871, Japan.
- KMID: 2455805
- DOI: http://doi.org/10.4196/kjpp.2019.23.5.295
Abstract
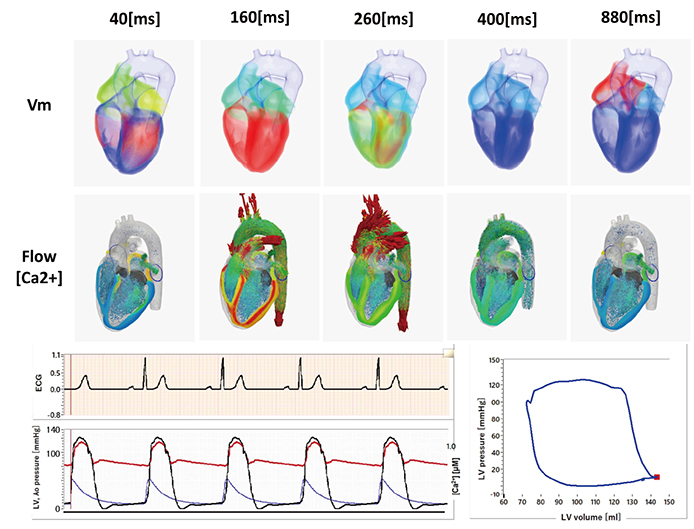
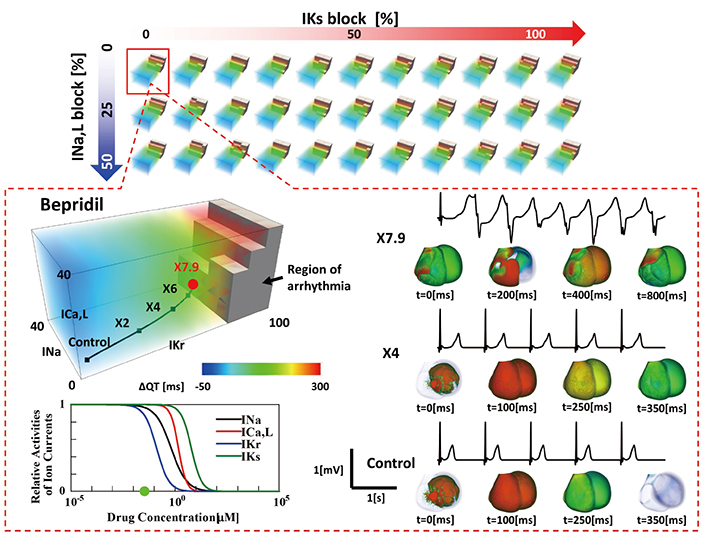
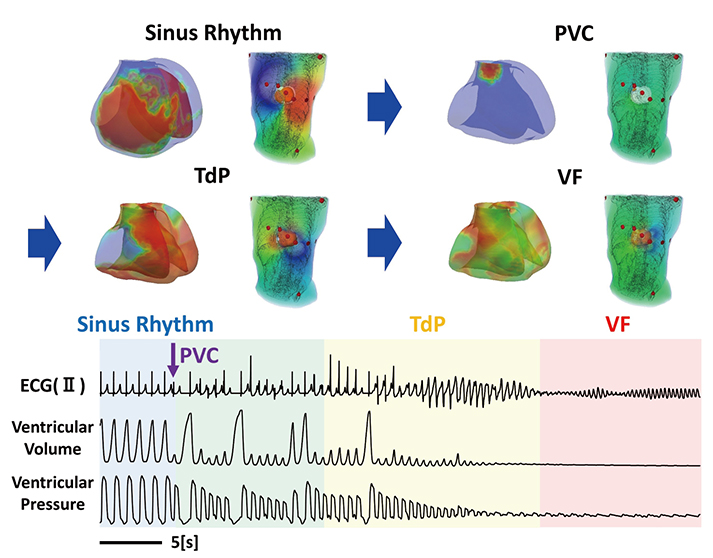
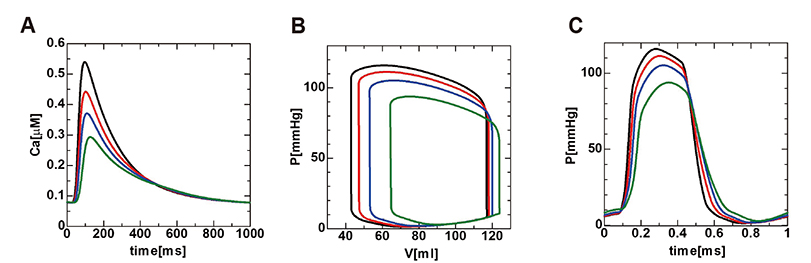
- A heart simulator, UT-Heart, is a finite element model of the human heart that can reproduce all the fundamental activities of the working heart, including propagation of excitation, contraction, and relaxation and generation of blood pressure and blood flow, based on the molecular aspects of the cardiac electrophysiology and excitation-contraction coupling. In this paper, we present a brief review of the practical use of UT-Heart. As an example, we focus on its application for predicting the effect of cardiac resynchronization therapy (CRT) and evaluating the proarrhythmic risk of drugs. Patient-specific, multiscale heart simulation successfully predicted the response to CRT by reproducing the complex pathophysiology of the heart. A proarrhythmic risk assessment system combining in vitro channel assays and in silico simulation of cardiac electrophysiology using UT-Heart successfully predicted druginduced arrhythmogenic risk. The assessment system was found to be reliable and efficient. We also developed a comprehensive hazard map on the various combinations of ion channel inhibitors. This in silico electrocardiogram database (now freely available at http://ut-heart.com/) can facilitate proarrhythmic risk assessment without the need to perform computationally expensive heart simulation. Based on these results, we conclude that the heart simulator, UT-Heart, could be a useful tool in clinical medicine and drug discovery.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Digital heart for life
Yin Hua Zhang
Korean J Physiol Pharmacol. 2019;23(5):291-293. doi: 10.4196/kjpp.2019.23.5.291.
Reference
-
1. Sugiura S, Washio T, Hatano A, Okada J, Watanabe H, Hisada T. Multi-scale simulations of cardiac electrophysiology and mechanics using the University of Tokyo heart simulator. Prog Biophys Mol Biol. 2012; 110:380–389.
Article2. Zhang Q, Hisada T. Analysis of fluid-structure interaction problems with structural buckling and large domain changes by ALE finite element method. Comput Methods Appl Mech Eng. 2001; 190:6341–6357.
Article3. Washio T, Hisada T, Watanabe H, Tezduyar TE. A robust preconditioner for fluid-structure interaction problems. Comput Methods Appl Mech Eng. 2005; 194:4027–4047.
Article4. Okada J, Washio T, Hisada T. Study of efficient homogenization algorithms for nonlinear problems. Comput Mech. 2010; 46:247–258.
Article5. Okada J, Sugiura S, Nishimura S, Hisada T. Three-dimensional simulation of calcium waves and contraction in cardiomyocytes using the finite element method. Am J Physiol Cell Physiol. 2005; 288:C510–C522.
Article6. Hatano A, Okada J, Washio T, Hisada T, Sugiura S. A three-dimensional simulation model of cardiomyocyte integrating excitationcontraction coupling and metabolism. Biophys J. 2011; 101:2601–2610.
Article7. Okada J, Sugiura S, Hisada T. Modeling for cardiac excitation propagation based on the Nernst-Planck equation and homogenization. Phys Rev E Stat Nonlin Soft Matter Phys. 2013; 87:062701.
Article8. Washio T, Okada J, Sugiura S, Hisada T. Approximation for cooperative interactions of a spatially-detailed cardiac sarcomere model. Cell Mol Bioeng. 2012; 5:113–126.
Article9. Washio T, Sugiura S, Kanada R, Okada J, Hisada T. Coupling Langevin dynamics with continuum mechanics: exposing the role of sarcomere stretch activation mechanisms to cardiac function. Front Physiol. 2018; 9:333.
Article10. Washio T, Yoneda K, Okada J, Kariya T, Sugiura S, Hisada T. Ventricular fiber optimization utilizing the branching structure. Int J Numer Method Biomed Eng. 2016; 32:e02753.
Article11. Katayama S, Umetani N, Sugiura S, Hisada T. The sinus of Valsalva relieves abnormal stress on aortic valve leaflets by facilitating smooth closure. J Thorac Cardiovasc Surg. 2008; 136:1528–1535.
Article12. Cui X, Washio T, Chono T, Baba H, Okada J, Sugiura S, Hisada T. Deformable regions of interest with multiple points for tissue tracking in echocardiography. Med Image Anal. 2017; 35:554–569.
Article13. Okada J, Washio T, Nakagawa M, Watanabe M, Kadooka Y, Kariya T, Yamashita H, Yamada Y, Momomura SI, Nagai R, Hisada T, Sugiura S. Absence of rapid propagation through the Purkinje network as a potential cause of line block in the human heart with left bundle branch block. Front Physiol. 2018; 9:56.
Article14. Okada J, Washio T, Maehara A, Momomura S, Sugiura S, Hisada T. Transmural and apicobasal gradients in repolarization contribute to T-wave genesis in human surface ECG. Am J Physiol Heart Circ Physiol. 2011; 301:H200–H208.
Article15. Washio T, Okada J, Hisada T. A parallel multilevel technique for solving the bidomain equation on a human heart with Purkinje fibers and a torso model. SIAM Rev. 2010; 52:717–743.
Article16. Watanabe H, Hisada T, Sugiura S, Okada J, Fukunari H. Computer simulation of blood flow, left ventricular wall motion and their interrelationship by fluid-structure interaction finite element method. JSME Int J. 2002; 45:1003–1012.
Article17. Watanabe H, Sugiura S, Kafuku H, Hisada T. Multiphysics simulation of left ventricular filling dynamics using fluid-structure interaction finite element method. Biophys J. 2004; 87:2074–2085.
Article18. Hosoi A, Washio T, Okada J, Kadooka Y, Nakajima K, Hisada T. A multi-scale heart simulation on massively parallel computers. Paper presented at: SC '10: Proceedings of the 2010 ACM/IEEE International Conference for High Performance Computing, Networking, Storage and Analysis. 2010 Nov 13–19; New Orleans, LA, USA. IEEE Computer Society;p. 1–11.19. Washio T, Okada J, Takahashi A, Yoneda K, Kadooka Y, Sugiura S, Hisada T. Multiscale heart simulation with cooperative stochastic cross-bridge dynamics and cellular structures. Multiscale Model Simul. 2013; 11:965–999.
Article20. T Hisada H Kurokawa N Oshida M Yamamoto T Washio J Okada H Watanabe S Sugiura . Japan Science and Technology Agency. Modeling device, program, computer-readable recording medium, and method of establishing correspondence. United States patent. US 8,554,491. 2013.21. T Washio T Hisada E Nunobiki J Okada S Sugiura . System for estimating membrane stress on arbitrarily-shaped curvilinear surface based on current configuration data. United States patent. US 9,241,639. 2016.22. Lee AWC, Costa CM, Strocchi M, Rinaldi CA, Niederer SA. Computational modeling for cardiac resynchronization therapy. J Cardiovasc Transl Res. 2018; 11:92–108.
Article23. Niederer SA, Lumens J, Trayanova NA. Computational models in cardiology. Nat Rev Cardiol. 2019; 16:100–111.
Article24. Okada J, Sasaki T, Washio T, Yamashita H, Kariya T, Imai Y, Nakagawa M, Kadooka Y, Nagai R, Hisada T, Sugiura S. Patient specific simulation of body surface ECG using the finite element method. Pacing Clin Electrophysiol. 2013; 36:309–321.
Article25. Panthee N, Okada J, Washio T, Mochizuki Y, Suzuki R, Koyama H, Ono M, Hisada T, Sugiura S. Tailor-made heart simulation predicts the effect of cardiac resynchronization therapy in a canine model of heart failure. Med Image Anal. 2016; 31:46–62.
Article26. Okada J, Washio T, Nakagawa M, Watanabe M, Kadooka Y, Kariya T, Yamashita H, Yamada Y, Momomura SI, Nagai R, Hisada T, Sugiura S. Multi-scale, tailor-made heart simulation can predict the effect of cardiac resynchronization therapy. J Mol Cell Cardiol. 2017; 108:17–23.
Article27. Chi KR. Regulatory watch: Speedy validation sought for new cardiotoxicity testing strategy. Nat Rev Drug Discov. 2013; 12:655.28. Chi KR. Revolution dawning in cardiotoxicity testing. Nat Rev Drug Discov. 2013; 12:565–567.
Article29. Gintant G, Sager PT, Stockbridge N. Evolution of strategies to improve preclinical cardiac safety testing. Nat Rev Drug Discov. 2016; 15:457–471.
Article30. Okada J, Yoshinaga T, Kurokawa J, Washio T, Furukawa T, Sawada K, Sugiura S, Hisada T. Screening system for drug-induced arrhythmogenic risk combining a patch clamp and heart simulator. Sci Adv. 2015; 1:e1400142.
Article31. Yonezawa A, Watanabe T, Yokokawa M, Sato M, Hirao K. Advanced Institute for Computational Science (AICS): Japanese national high-performance computing research institute and its 10-petaflops supercomputer “K”. Paper presented at: Proceedings of 2011 International Conference for High Performance Computing, Networking, Storage and Analysis. 2011 Nov 12–18; Seatle, WA, USA.32. Okada J, Yoshinaga T, Kurokawa J, Washio T, Furukawa T, Sawada K, Sugiura S, Hisada T. Arrhythmic hazard map for a 3D wholeventricle model under multiple ion channel block. Br J Pharmacol. 2018; 175:3435–3452.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Broad-beam CT Image Reconstruction from Simulator Images
- Clinical application of tape-recorder and telemetry system for analysis of fetal heart rate
- Pharmacological Treatment of Heart Failure
- Comments on ‘Development of colonic stent simulator using three-dimensional printing technique: a simulator development study in Korea’
- An Integrative Model of the Cardiovascular System Coupling Heart Cellular Mechanics with Arterial Network Hemodynamics