Anesth Pain Med.
2019 Jul;14(3):299-304. 10.17085/apm.2019.14.3.299.
Sugammadex associated profound bradycardia and sustained hypotension in patient with the slow recovery of neuromuscular blockade: A case report
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chosun University Hospital, Gwangju, Korea. mdmole@chosun.ac.kr
- KMID: 2454815
- DOI: http://doi.org/10.17085/apm.2019.14.3.299
Abstract
- BACKGROUND
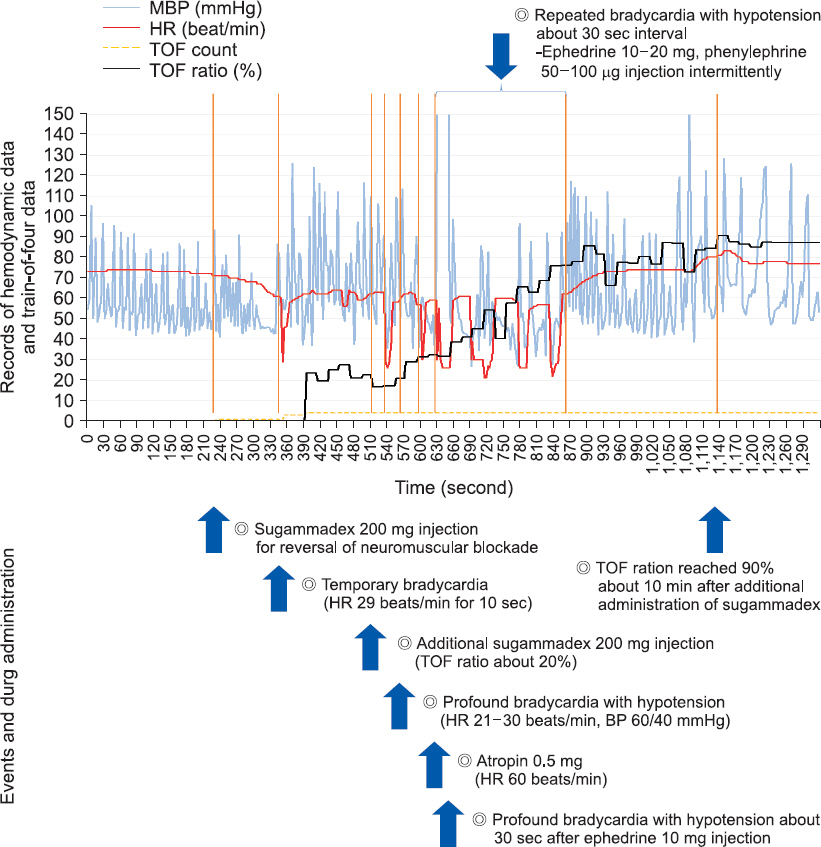
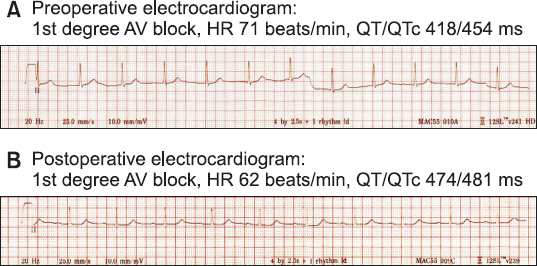
New complications associated with sugammadex have been increased since its widespread use. We report a case of an 80-year-old male who experienced profound bradycardia and sustained hypotension after administration of sugammadex. CASE: Following administration of 200 mg sugammadex after laparoscopic cholecystectomy, sudden bradycardia (29 beats/min) developed for 10 seconds and his train-of-four (TOF) ratio remained at 0.2 for 5 min. An additional 200 mg sugammadex was administered and profound bradycardia (21-30 beats/min) and hypotension (60/40 mmHg) developed. Atropine at 0.5 mg was administered, but the effect lasted only 30 s. Profound bradycardia occurred four more times at 30 s intervals, and ephedrine and phenylephrine were injected intermittently to increase the patient's heart rate and blood pressure. The TOF ratio became 0.9 about 10 min after administration of additional sugammadex.
CONCLUSIONS
Awareness must be heightened regarding the possibility of sugammadexinduced bradycardia and hypotension, and more attention should be paid to patients with slow recovery times following muscle relaxation, despite the use of sugammadex.
MeSH Terms
Figure
Cited by 1 articles
-
Adverse events of sugammadex that occurred in a Korean population
Woong Han, Jong Min Lee, Dong Ho Park, Chia An Lee, Chang Yeong Jeong, Hong Seuk Yang
Anesth Pain Med. 2022;17(2):191-198. doi: 10.17085/apm.21096.
Reference
-
1. Godai K, Hasegawa-Moriyama M, Kuniyoshi T, Kakoi T, Ikoma K, Isowaki S, et al. Three cases of suspected sugammadex-induced hypersensitivity reactions. Br J Anaesth. 2012; 109:216–8. DOI: 10.1093/bja/aes137. PMID: 22617091.2. Kim YH. Sugammadex:watch out for new side effects. Korean J Anesthesiol. 2016; 69:427–8. DOI: 10.4097/kjae.2016.69.5.427. PMID: 27703621. PMCID: PMC5047976.3. Sanoja IA, Toth KS. Profound bradycardia and cardiac arrest after sugammadex administration in a previously healthy patient: a case report. A A Pract. 2019; 12:22–4. DOI: 10.1213/XAA.0000000000000834. PMID: 30004912.4. Bhavani SS. Severe bradycardia and asystole after sugammadex. Br J Anaesth. 2018; 121:95–6. DOI: 10.1016/j.bja.2018.02.036. PMID: 29935601.5. Hunter JM, Naguib M. Sugammadex-induced bradycardia and asystole: how great is the risk? Br J Anaesth. 2018; 121:8–12. DOI: 10.1016/j.bja.2018.03.003. PMID: 29935599.6. Shin HS, Kim YR, Kim JA, Chung IS. Profound bradycardia and hypotension after sugammadex administration. J Clin Anesth Manag. 2017; 2:1–3. DOI: 10.16966/2470-9956.126.7. Simons FE, Ardusso LR, Bilò MB, Dimov V, Ebisawa M, El-Gamal YM, et al. 2012 update:World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012; 12:389–99. DOI: 10.1097/ACI.0b013e328355b7e4. PMID: 22744267.8. Pühringer FK, Rex C, Sielenkämper AW, Claudius C, Larsen PB, Prins ME, et al. Reversal of profound, high-dose rocuronium-induced neuromuscular blockade by sugammadex at two different time points:an international, multicenter, randomized, dosefinding, safety assessor-blinded, phase II trial. Anesthesiology. 2008; 109:188–97. DOI: 10.1097/ALN.0b013e31817f5bc7. PMID: 18648227.9. Dahl V, Pendeville PE, Hollmann MW, Heier T, Abels EA, Blobner M. Safety and efficacy of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in cardiac patients undergoing noncardiac surgery. Eur J Anaesthesiol. 2009; 26:874–84. DOI: 10.1097/EJA.0b013e32832c605b. PMID: 19455040.10. Osaka Y, Shimada N, Satou M, Masuda T, Ando T, Kozono Y, et al. A case of atrioventricular block (Wenckebach type) induced by sugammadex. J Anesth. 2012; 26:627–8. DOI: 10.1007/s00540-012-1390-x. PMID: 22526433.11. Saito I, Osaka Y, Shimada M. Transient third-degree AV block following sugammadex. J Anesth. 2015; 29:641. DOI: 10.1007/s00540-015-1980-5. PMID: 25672653.12. King A, Naguib A, Tobias JD. Bradycardia in a pediatric heart transplant recipient:is it the sugammadex? J Pediatr Pharmacol Ther. 2017; 22:378–81. DOI: 10.5863/1551-6776-22.5.378. PMID: 29042841. PMCID: PMC5640307.13. Booij LH, van Egmond J, Driessen JJ, de Boer HD. In vivo animal studies with sugammadex. Anaesthesia. 2009; 64(Suppl 1):38–44. DOI: 10.1111/j.1365-2044.2008.05869.x. PMID: 19222430.14. Kim SH, Lee SM, Kim YK, Park SY, Lee JH, Cho SH, et al. Effects of prophylactic ramosetron and ondansetron on corrected QT interval during general anesthesia. J Clin Anesth. 2014; 26:511–6. DOI: 10.1016/j.jclinane.2014.02.011. PMID: 25439413.15. Lee S. What anesthesiologists ask to know and should know about the neuromuscular monitoring: an updated review. Anesth Pain Med. 2017; 12:1–8. DOI: 10.17085/apm.2017.12.1.1.