Cancer Res Treat.
2016 Apr;48(2):567-573. 10.4143/crt.2015.195.
Endoscopic Criteria for Evaluating Tumor Stage after Preoperative Chemoradiation Therapy in Locally Advanced Rectal Cancer
- Affiliations
-
- 1Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. gsgsbal@ncc.re.kr
- KMID: 2454334
- DOI: http://doi.org/10.4143/crt.2015.195
Abstract
- PURPOSE
Local excision may be an another option for selected patients with markedly down-staged rectal cancer after preoperative chemoradiation therapy (CRT), and proper evaluation of post-CRT tumor stage (ypT) is essential prior to local excision of these tumors. This study was designed to determine the correlations between endoscopic findings and ypT of rectal cancer.
MATERIALS AND METHODS
In this study, 481 patients with locally advanced rectal cancer who underwent preoperative CRT followed by surgical resection between 2004 and 2013 at a single institution were evaluated retrospectively. Pathological good response (p-GR) was defined as ypT ≤ 1, and pathological minimal or no response (p-MR) as ypT ≥ 2. The patients were randomly classified according to two groups, a testing (n=193) and a validation (n=288) group. Endoscopic criteria were determined from endoscopic findings and ypT in the testing group and used in classifying patients in the validation group as achieving or not achieving p-GR.
RESULTS
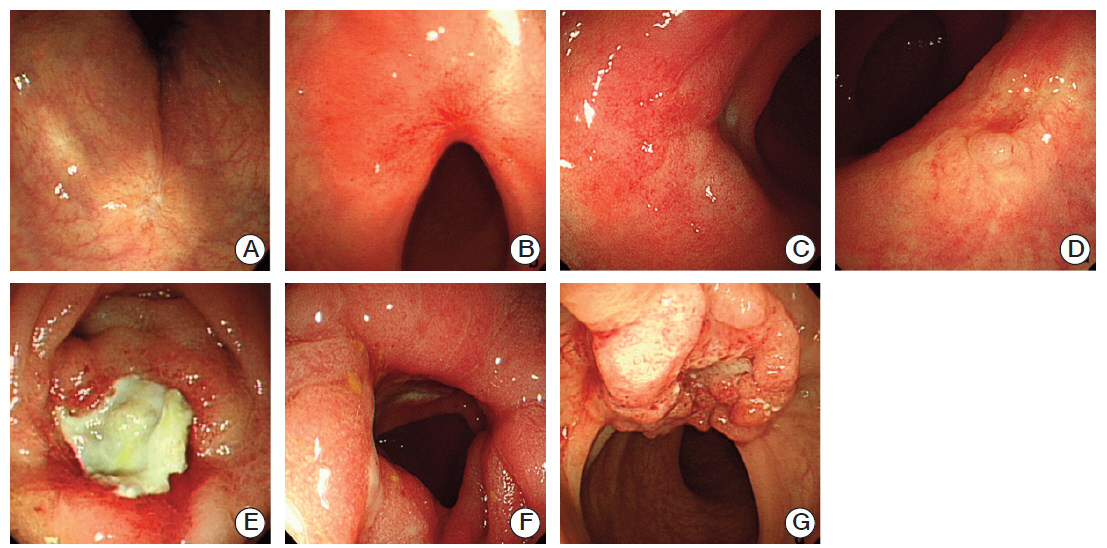
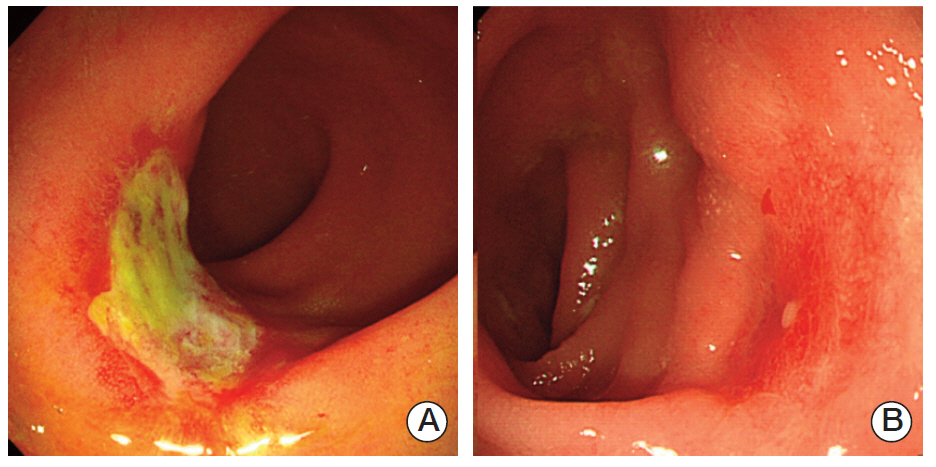
Based on findings in the testing group, the endoscopic criteria for p-GR included scarring, telangiectasia, and erythema, whereas criteria for p-MR included nodules, ulcers, strictures, and remnant tumors. In the validation group, the kappa statistic was 0.965 (p < 0.001), and the sensitivity, specificity, positive predictive value, and negative predictive value were 0.362, 0.963, 0.654, and 0.885, respectively.
CONCLUSION
The endoscopic criteria presented are easily applicable for evaluation of ypT after preoperative CRT for rectal cancer. These criteria may be used for selection of patients for local excision of down-staged rectal tumors, because patients with p-MR could be easily ruled out.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351:1731–40.
Article2. Sanghera P, Wong DW, McConkey CC, Geh JI, Hartley A. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol). 2008; 20:176–83.
Article3. Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013; 144:551–9.
Article4. Suh JH, Han KS, Kim BC, Hong CW, Sohn DK, Chang HJ, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy. 2012; 44:590–5.
Article5. Lezoche E, Guerrieri M, Paganini AM, Baldarelli M, De Sanctis A, Lezoche G. Long-term results in patients with T2-3 N0 dis-tal rectal cancer undergoing radiotherapy before transanal endoscopic microsurgery. Br J Surg. 2005; 92:1546–52.
Article6. Kundel Y, Brenner R, Purim O, Peled N, Idelevich E, Fenig E, et al. Is local excision after complete pathological response to neoadjuvant chemoradiation for rectal cancer an acceptable treatment option? Dis Colon Rectum. 2010; 53:1624–31.
Article7. Yeo SG, Kim DY, Kim TH, Kim SY, Chang HJ, Park JW, et al. Local excision following pre-operative chemoradiotherapy-induced downstaging for selected cT3 distal rectal cancer. Jpn J Clin Oncol. 2010; 40:754–60.
Article8. Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012; 99:1211–8.
Article9. Issa N, Murninkas A, Powsner E, Dreznick Z. Long-term outcome of local excision after complete pathological response to neoadjuvant chemoradiation therapy for rectal cancer. World J Surg. 2012; 36:2481–7.
Article10. Gagliardi G, Newton TR, Bailey HR. Local excision of rectal cancer followed by radical surgery because of poor prognostic features does not compromise the long term oncologic outcome. Colorectal Dis. 2013; 15:e659–64.
Article11. Pucciarelli S, De Paoli A, Guerrieri M, La Torre G, Maretto I, De Marchi F, et al. Local excision after preoperative chemoradiotherapy for rectal cancer: results of a multicenter phase II clinical trial. Dis Colon Rectum. 2013; 56:1349–56.12. Kuo LJ, Chiou JF, Tai CJ, Chang CC, Kung CH, Lin SE, et al. Can we predict pathologic complete response before surgery for locally advanced rectal cancer treated with preoperative chemoradiation therapy? Int J Colorectal Dis. 2012; 27:613–21.
Article13. Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010; 53:1692–8.
Article14. Suzuki T, Sadahiro S, Tanaka A, Okada K, Okamura H, Machida T. Prediction of histologic regression on the basis of colonoscopic findings in patients with locally advanced middle and lower rectal cancer who receive preoperative chemoradiotherapy. Tokai J Exp Clin Med. 2011; 36:100–5.15. Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002; 194:131–5.16. Guillem JG, Chessin DB, Shia J, Moore HG, Mazumdar M, Bernard B, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol. 2005; 23:3475–9.
Article17. Pastor C, Subtil JC, Sola J, Baixauli J, Beorlegui C, Arbea L, et al. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum. 2011; 54:1141–6.
Article18. Duldulao MP, Lee W, Streja L, Chu P, Li W, Chen Z, et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum. 2013; 56:142–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Outcome of Preoperative Chemoradiation to Locally Advanced Rectal Cancer
- How to Achieve a Higher Pathologic Complete Response in Patients With Locally Advanced Rectal Cancer Who Receive Preoperative Chemoradiation Therapy
- The Effects and Surgical Morbidity of Preoperative Combined Chemoradiotherapy for Locally Advanced Rectal Cancer
- Imaging Diagnosis of Locally Advanced Rectal Cancer: Tumor Staging before and after Preoperative Chemoradiotherapy
- The Effect of Preoperative Concurrent Chemoradiation in Locally Advanced Rectal Cancer