Cancer Res Treat.
2019 Jul;51(3):1086-1097. 10.4143/crt.2018.537.
Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. sehyunkim@snubh.org
- 2Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- KMID: 2454300
- DOI: http://doi.org/10.4143/crt.2018.537
Abstract
- PURPOSE
Programmed death-1 (PD-1)/PD-1 ligand (PD-L1) axis blockades have revolutionized the treatment of advanced non-small cell lung cancer (NSCLC). We assessed the effect of platinum-based chemotherapy on tumor PD-L1 expression and its clinical implications.
MATERIALS AND METHODS
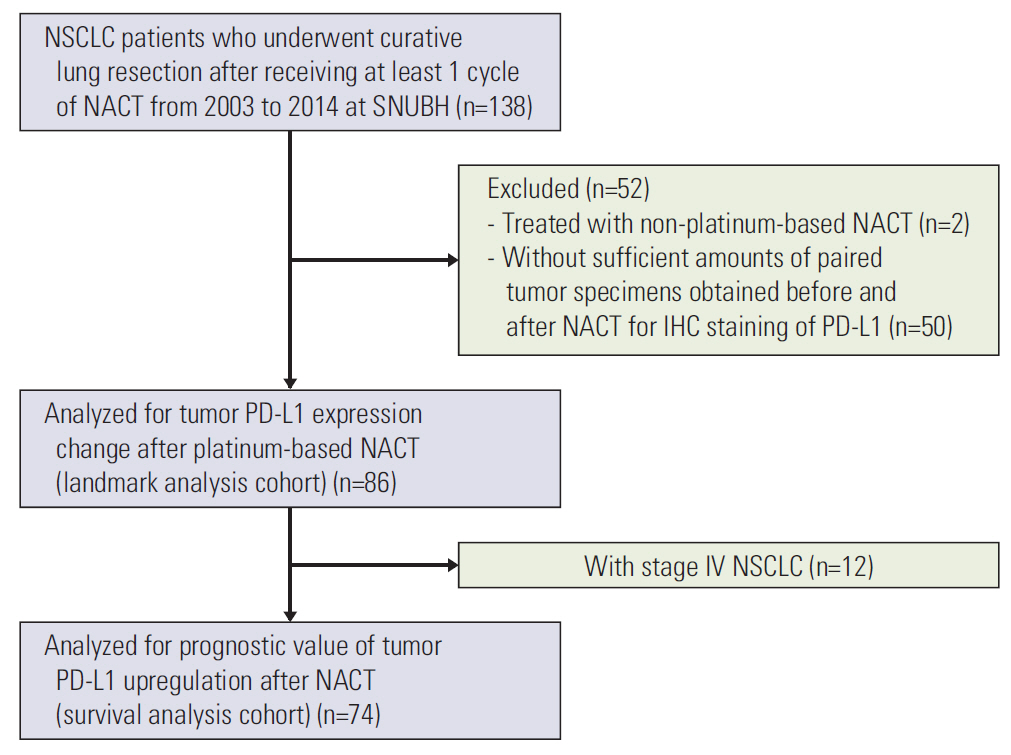
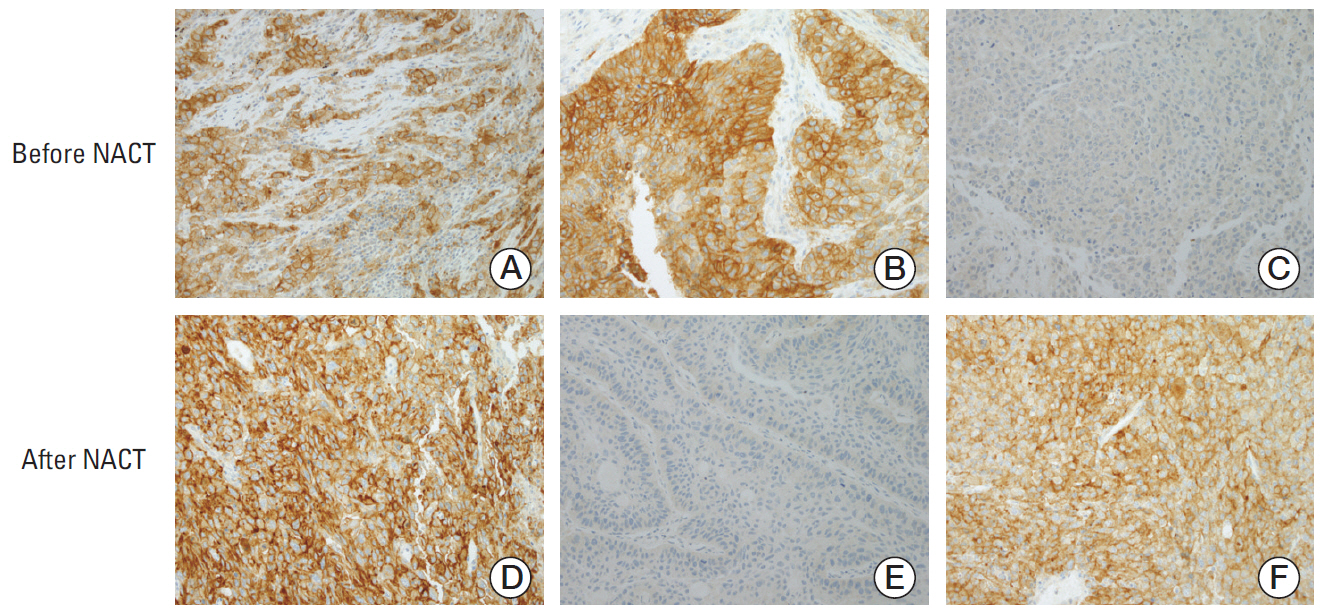
We used immunohistochemistry to retrospectively evaluate the percentage of tumor cells with membranous PD-L1 staining (tumor proportion score) in paired tumor specimens obtained before and after platinum-based neoadjuvant chemotherapy (NACT) in 86 patients with NSCLC. We analyzed the correlation between the change in PD-L1 tumor proportion score and clinicopathologic characteristics, response to NACT, and survival.
RESULTS
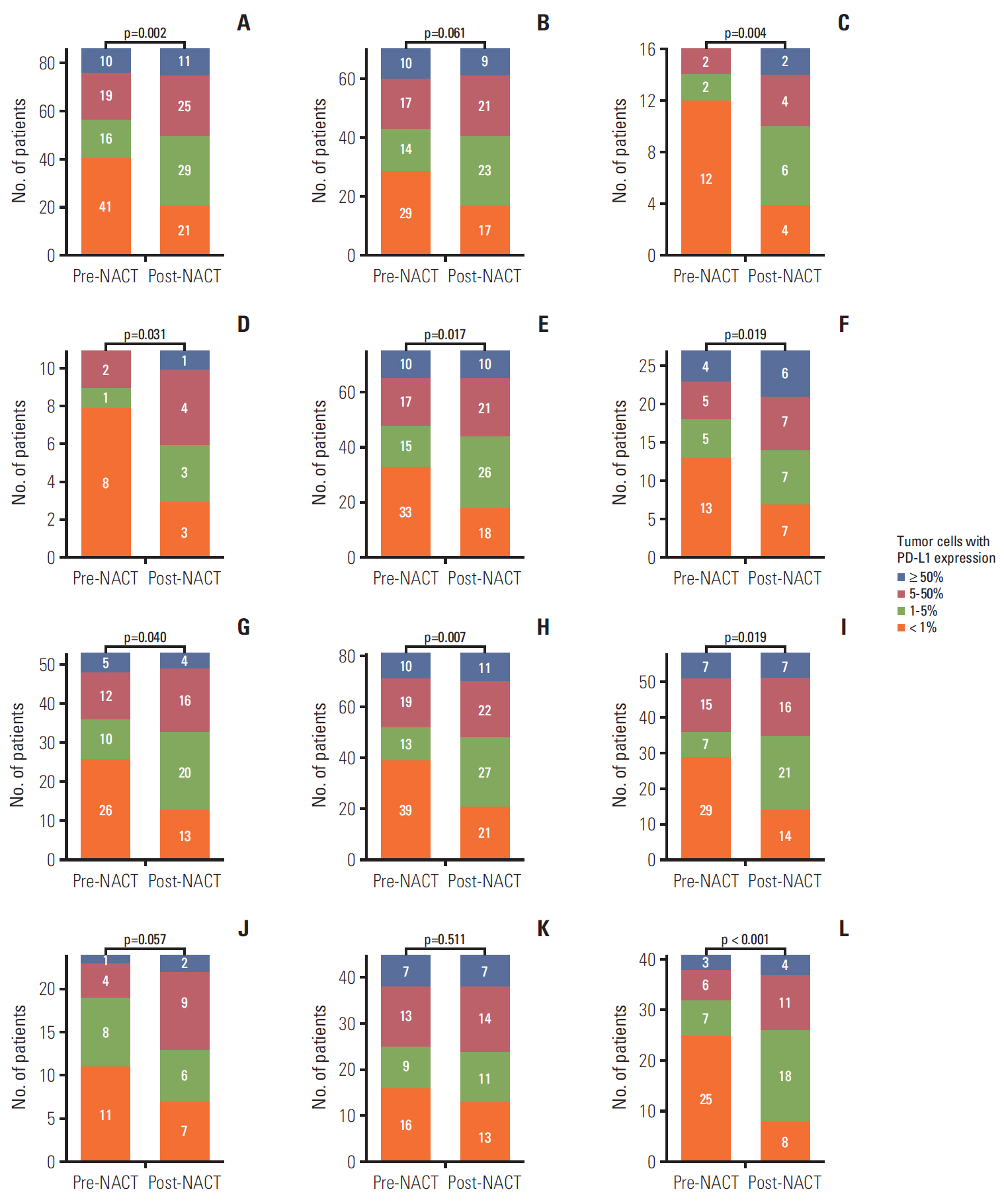
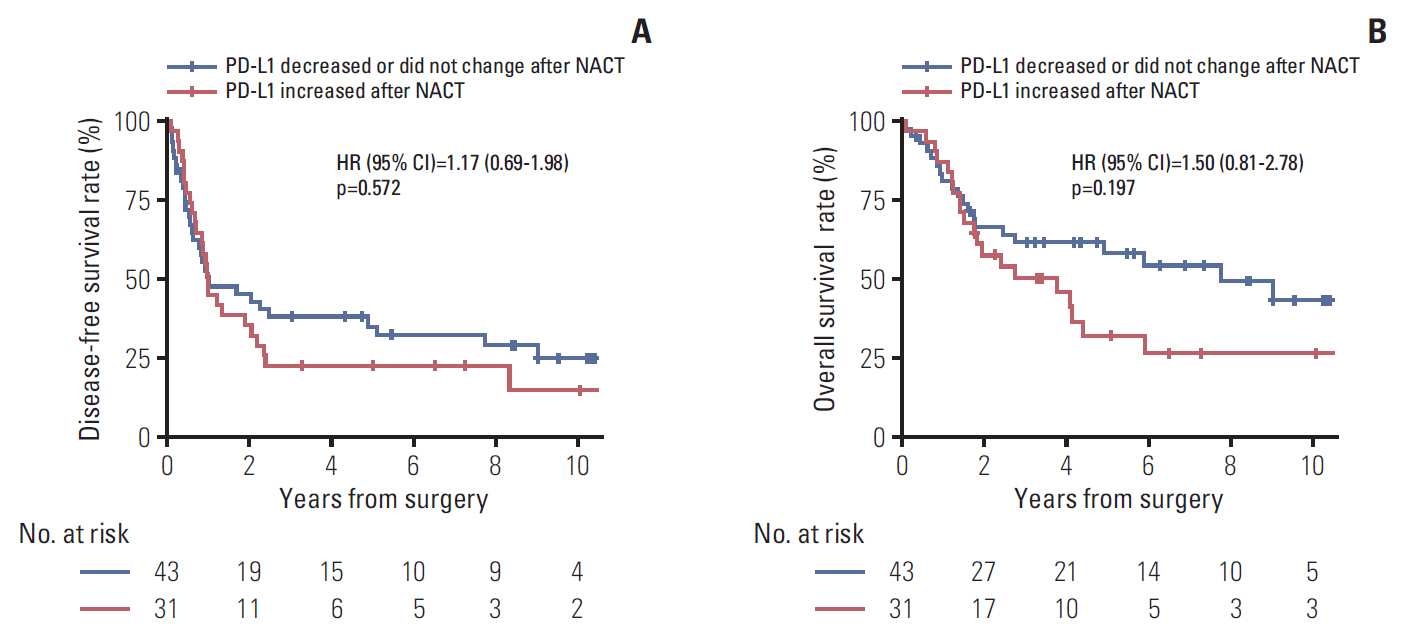
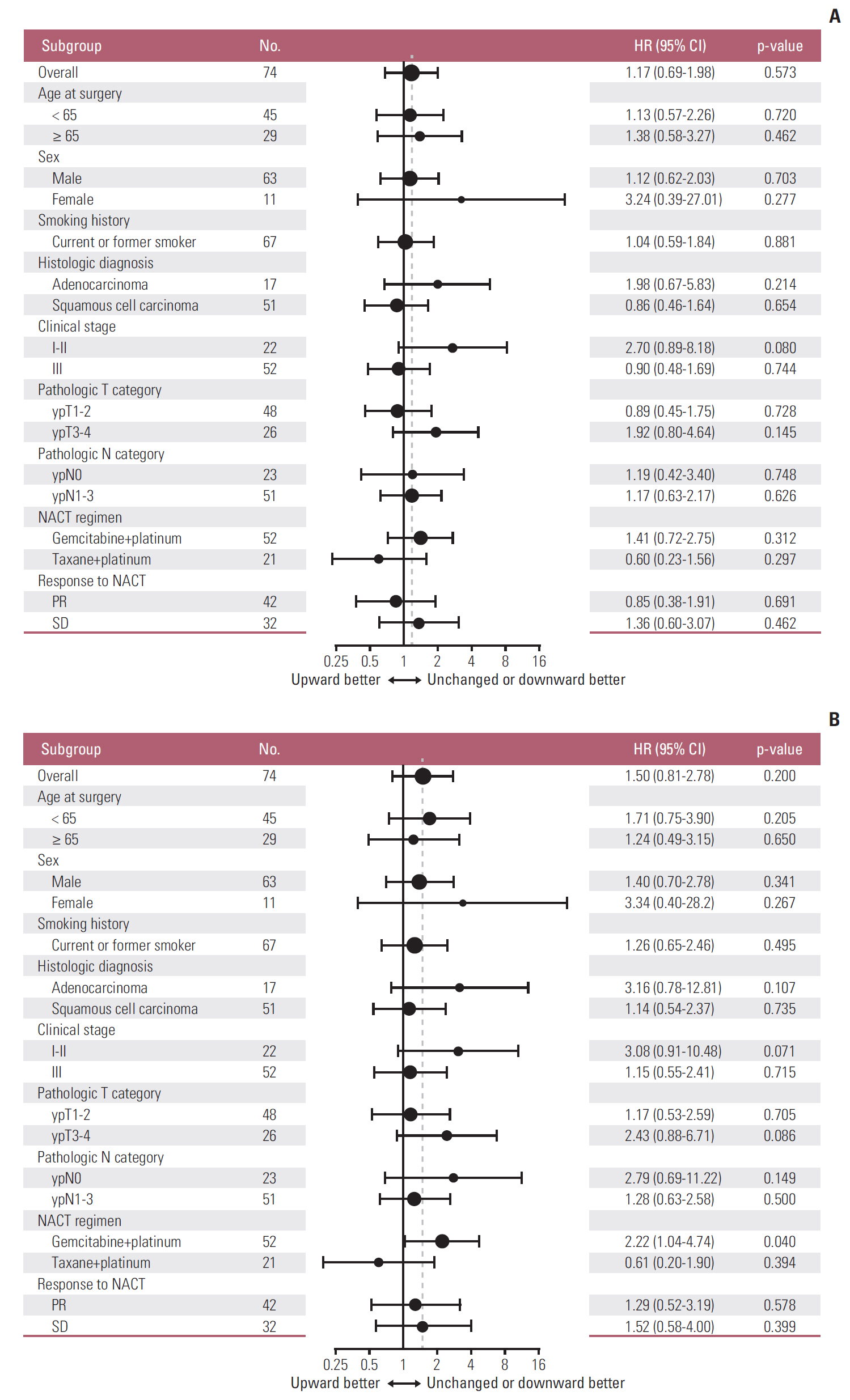
The PD-L1 tumor proportion score increased in a significant proportion of patients with NSCLC after platinum-based NACT (Wilcoxon signed-rank test, p=0.002). That pattern was consistent across clinically defined subgroups except for patients with partial response to NACT. Tumors from 26 patients (30.2%) were PD-L1"’negative before NACT but PD-L1-positive after NACT, whereas the reverse pattern occurred in six patients (7%) (McNemar's test, p < 0.001). Increase in PD-L1 tumor proportion score was significantly associated with lack of response to NACT (Fisher exact test, p=0.015). There was a tendency, albeit not statistically significant, for patients with an increase in PD-L1 tumor proportion score to have shorter survival.
CONCLUSION
Tumor PD-L1 expression increased after platinum-based NACT in a significant proportion of patients with NSCLC. Increase in tumor PD-L1 expression may predict poor clinical outcome.
MeSH Terms
Figure
Cited by 1 articles
-
PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
Hyojin Kim, Jin-Haeng Chung
J Pathol Transl Med. 2019;53(4):199-206. doi: 10.4132/jptm.2019.04.24.
Reference
-
References
1. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016; 375:1823–33.
Article2. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018; 378:2078–92.
Article3. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387:1540–50.
Article4. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015; 373:123–35.
Article5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–39.
Article6. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017; 389:255–65.
Article7. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist. 2016; 21:643–50.
Article8. Parra ER, Villalobos P, Behrens C, Jiang M, Pataer A, Swisher SG, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer. 2018; 6:48.
Article9. Sheng J, Fang W, Yu J, Chen N, Zhan J, Ma Y, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep. 2016; 6:20090.
Article10. Fujimoto D, Uehara K, Sato Y, Sakanoue I, Ito M, Teraoka S, et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep. 2017; 7:11373.
Article11. Song Z, Yu X, Zhang Y. Altered expression of programmed death-ligand 1 after neo-adjuvant chemotherapy in patients with lung squamous cell carcinoma. Lung Cancer. 2016; 99:166–71.
Article12. Rojko L, Reiniger L, Teglasi V, Fabian K, Pipek O, Vagvolgyi A, et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J Cancer Res Clin Oncol. 2018; 144:1219–26.
Article13. Zhang P, Ma Y, Lv C, Huang M, Li M, Dong B, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci. 2016; 107:1563–71.
Article14. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017; 15:504–35.
Article15. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007; 7:573–84.
Article16. Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. 2014; 20:2831–7.
Article17. Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011; 121:3100–8.
Article18. Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016; 34:2969–79.
Article19. Liu SV, Camidge DR, Gettinger SN, Giaccone G, Heist RS, Hodi FS, et al. Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non-small-cell lung cancer. Eur J Cancer. 2018; 101:114–22.
Article20. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007; 2:706–14.
Article21. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014; 515:563–7.
Article22. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016; 387:1837–46.
Article23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article24. Tsao MS, Le Teuff G, Shepherd FA, Landais C, Hainaut P, Filipits M, et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol. 2017; 28:882–9.
Article25. Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016; 11:1003–11.
Article26. Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015; 41:450–6.
Article27. Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016; 55:7–14.
Article28. Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015; 89:181–8.
Article29. O'Brien ME, Hasan B, Dafni U, Menis J, Peters S, De Waele M, et al. EORTC-ETOP randomized, phase 3 trial with anti-PD-1 monoclonal antibody pembrolizumab versus placebo for patients with early stage non-small cell lung cancer (NSCLC) after resection and standard adjuvant chemotherapy: PEARLS (NCT02504372). J Clin Oncol. 2016; 34(15 Suppl):TPS8571.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PD-L1 Testing in Non-small Cell Lung Cancer: Past, Present, and Future
- Programmed death-ligand 1 expression and tumor-infiltrating lymphocytes in non-small cell lung cancer: association with clinicopathologic parameters
- Temporal evolution of programmed death-ligand 1 expression in patients with non-small cell lung cancer
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- Impact of TTF-1 Expression on the Prognostic Prediction of Patients with Non–Small Cell Lung Cancer with PD-L1 Expression Levels of 1% to 49%, Treated with Chemotherapy vs. Chemoimmunotherapy: A Multicenter, Retrospective Study