Yonsei Med J.
2012 Sep;53(5):1049-1053.
Two Cases of Wernicke's Encephalopathy in Young Age Patients Receiving Allogeneic Hematopoietic Stem Cell Transplantation
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2Division of Pediatric Hematology and Oncology, Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea. cj@yuhs.ac
Abstract
- Wernicke's encephalopathy is an acute neurolopsychiatric syndrome caused by thiamine deficiency, and classically presents with the triad of opthalmopathy, ataxia and altered mentality. Both prolonged total parenteral nutrition and reduced oral intake can induce Wernicke's encephalopathy during hematopoietic stem cell transplantation (HSCT). Although early treatment is important for recovery from Wernicke's encephalopathy, the vague symptoms and characteristics hinder early diagnosis. Furthermore, Wernicke's encephalopathy is not infrequent and can develop at any age during HSCT. Herein, we present two young patients developing Wernicke's encephalopathy during HSCT.
MeSH Terms
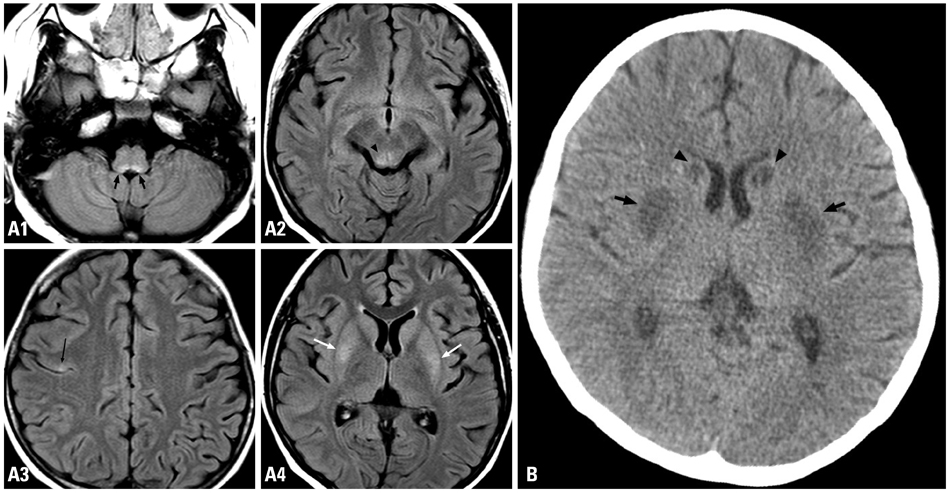
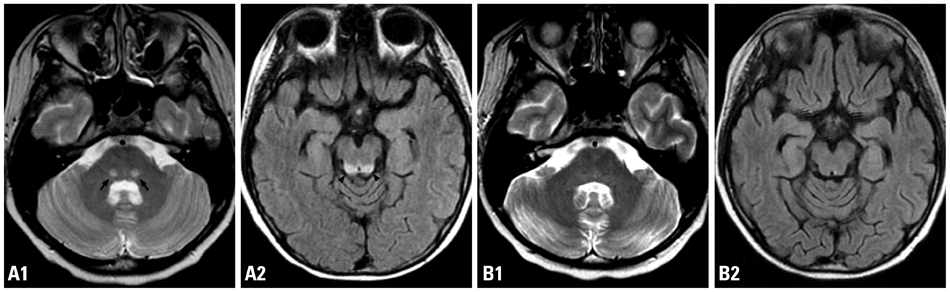
Figure
Reference
-
1. Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007. 6:442–455.
Article2. Thomson AD, Marshall EJ. The natural history and pathophysiology of Wernicke's Encephalopathy and Korsakoff's Psychosis. Alcohol Alcohol. 2006. 41:151–158.
Article3. Davis RE, Icke GC. Clinical chemistry of thiamin. Adv Clin Chem. 1983. 23:93–140.
Article4. Francini-Pesenti F, Brocadello F, Manara R, Santelli L, Laroni A, Caregaro L. Wernicke's syndrome during parenteral feeding: not an unusual complication. Nutrition. 2009. 25:142–146.
Article5. Bleggi-Torres LF, de Medeiros BC, Ogasawara VS, Loddo G, Zanis Neto J, Pasquini R, et al. Iatrogenic Wernicke's encephalopathy in allogeneic bone marrow transplantation: a study of eight cases. Bone Marrow Transplant. 1997. 20:391–395.
Article6. Torvik A. Wernicke's encephalopathy--prevalence and clinical spectrum. Alcohol Alcohol Suppl. 1991. 1:381–384.7. Pearce JM. Wernicke-Korsakoff encephalopathy. Eur Neurol. 2008. 59:101–104.
Article8. Day E, Bentham P, Callaghan R, Kuruvilla T, George S. Thiamine for Wernicke-Korsakoff Syndrome in people at risk from alcohol abuse. Cochrane Database Syst Rev. 2004. CD004033.
Article9. Hahn JS, Berquist W, Alcorn DM, Chamberlain L, Bass D. Wernicke encephalopathy and beriberi during total parenteral nutrition attributable to multivitamin infusion shortage. Pediatrics. 1998. 101:E10.
Article10. Baek JH, Sohn SK, Kim DH, Kim JG, Lee HW, Park SP, et al. Wernicke's encephalopathy after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005. 35:829–830.
Article11. Choi YJ, Park SJ, Kim JS, Kang EJ, Choi CW, Kim BS. Wernicke's encephalopathy following allogeneic hematopoietic stem cell transplantation. Korean J Hematol. 2010. 45:279–281.
Article12. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008. 29:1036–1042.
Article13. Bleggi-Torres LF, de Medeiros BC, Werner B, Neto JZ, Loddo G, Pasquini R, et al. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000. 25:301–307.
Article14. Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Gallucci M, Carollo C, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009. 30:171–176.
Article15. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007. 28:1652–1658.
Article16. Kondo K, Fujiwara M, Murase M, Kodera Y, Akiyama S, Ito K, et al. Severe acute metabolic acidosis and Wernicke's encephalopathy following chemotherapy with 5-fluorouracil and cisplatin: case report and review of the literature. Jpn J Clin Oncol. 1996. 26:234–236.
Article17. Koenig H, Patel A. Biochemical basis for fluorouracil neurotoxicity. The role of Krebs cycle inhibition by fluoroacetate. Arch Neurol. 1970. 23:155–160.18. Boros LG, Brandes JL, Lee WN, Cascante M, Puigjaner J, Revesz E, et al. Thiamine supplementation to cancer patients: a double edged sword. Anticancer Res. 1998. 18:595–602.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Wernicke's encephalopathy following allogeneic hematopoietic stem cell transplantation
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
- Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes
- Two Cases of Wernicke's Encephalopathy with Hyperemesis Gravidarum
- Comparison of Quality of Life of Autologous and Allogeneic Hematopoietic Stem Cell Transplantation Recipients