Yonsei Med J.
2012 Sep;53(5):944-951.
Prognostic Factors and Characteristics of Pancreatic Neuroendocrine Tumors: Single Center Experience
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine and Yonsei Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea. sysong@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Pancreatic neuroendocrine tumors (PNET) are a rare subgroup of tumors. For PNETs, the predictive factors for survival and prognosis are not well known. The purpose of our study was to evaluate the predictive factors for survival and disease progression in PNETs.
MATERIALS AND METHODS
We retrospectively analyzed 37 patients who were diagnosed with PNET at Severance Hospital between November 2005 and March 2010. Prognostic factors for survival and disease progression were evaluated using the Kaplan-Meier method.
RESULTS
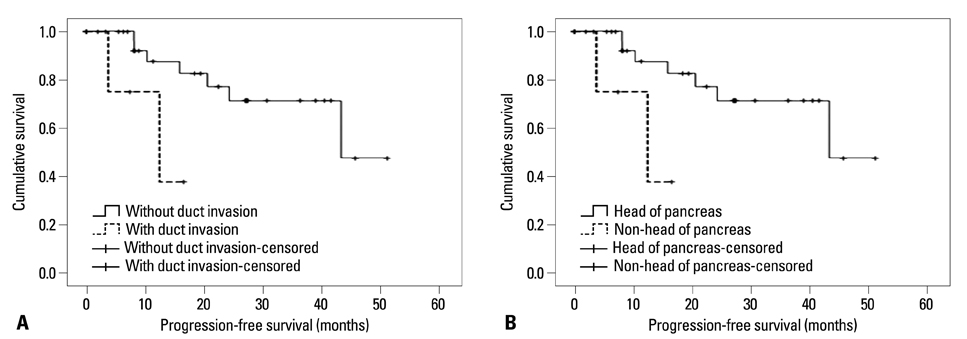
The mean age of the patients was 50.0+/-15.0 years. Eight cases (21.6%) were described as functioning tumors and 29 cases (78.4%) as non-functioning tumors. In univariate analysis of clinical factors, patients with liver metastasis (p=0.002), without resection of primary tumors (p=0.002), or American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) stage III/IV (p=0.002) were more likely to demonstrate shorter overall survival (OS). Patients with bile duct or pancreatic duct invasion (p=0.031), sized-lesions larger than 20 mm (p=0.036), liver metastasis (p=0.020), distant metastasis (p=0.005), lymph node metastasis (p=0.009) or without resection of primary tumors (p=0.020) were more likely to demonstrate shorter progression-free survival (PFS). In multivariate analysis of clinical factors, bile duct or pancreatic duct invasion [p=0.010, hazard ratio (HR)=95.046] and tumor location (non-head of pancreas) (p=0.036, HR=7.381) were confirmed as independent factors for predicting shorter PFS.
CONCLUSION
Patients with liver metastasis or without resection of primary tumors were more likely to demonstrate shorter OS. Patients with bile duct or pancreatic duct invasion or tumors located at body or tail of pancreas were more likely to demonstrate shorter PFS.
Keyword
MeSH Terms
Figure
Reference
-
1. Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005. 19:753–781.
Article2. Eriksson B, Oberg K. Neuroendocrine tumours of the pancreas. Br J Surg. 2000. 87:129–131.
Article3. Ong SL, Garcea G, Pollard CA, Furness PN, Steward WP, Rajesh A, et al. A fuller understanding of pancreatic neuroendocrine tumours combined with aggressive management improves outcome. Pancreatology. 2009. 9:583–600.
Article4. Ehehalt F, Saeger HD, Schmidt CM, Grützmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009. 14:456–467.
Article5. Norton JA, Warren RS, Kelly MG, Zuraek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003. 134:1057–1063.
Article6. Chen H, Hardacre JM, Uzar A, Cameron JL, Choti MA. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998. 187:88–92.
Article7. Han JH, Kim MH, Moon SH, Park SJ, Park DH, Lee SS, et al. [Clinical characteristics and malignant predictive factors of pancreatic neuroendocrine tumors]. Korean J Gastroenterol. 2009. 53:98–105.8. Paik WH, Yoon YB, Lee SH, Park JK, Woo SM, Yang KY, et al. [Pancreatic endocrine tumors: clinical manifestations and predictive factors associated with survival]. Korean J Gastroenterol. 2008. 52:171–178.9. Kang TW, Lee KT, Ryu MK, Moon W, Lee SS, Lee SY, et al. [Clinical features of neuroendocrine tumor of the pancreas: single center study]. Korean J Gastroenterol. 2006. 48:112–118.10. Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005. 12:1083–1092.
Article11. Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008. 135:1469–1492.
Article12. Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, et al. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997. 82:2622–2628.
Article13. Zimmer T, Stölzel U, Bäder M, Koppenhagen K, Hamm B, Buhr H, et al. Endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localisation of insulinomas and gastrinomas. Gut. 1996. 39:562–568.
Article14. Pape UF, Jann H, Müller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008. 113:256–265.
Article15. Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006. 449:395–401.
Article16. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010. 17:1471–1474.
Article17. Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009. 115:741–751.18. Hodul PJ, Strosberg JR, Kvols LK. Aggressive surgical resection in the management of pancreatic neuroendocrine tumors: when is it indicated? Cancer Control. 2008. 15:314–321.
Article19. Liu H, Zhang SZ, Wu YL, Fang HQ, Li JT, Sheng HW, et al. Diagnosis and surgical treatment of pancreatic endocrine tumors in 36 patients: a single-center report. Chin Med J (Engl). 2007. 120:1487–1490.
Article20. Kaemmerer D, Prasad V, Daffner W, Hörsch D, Klöppel G, Hommann M, et al. Neoadjuvant peptide receptor radionuclide therapy for an inoperable neuroendocrine pancreatic tumor. World J Gastroenterol. 2009. 15:5867–5870.
Article21. Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010. 16:2963–2970.
Article22. Massironi S, Sciola V, Peracchi M, Ciafardini C, Spampatti MP, Conte D. Neuroendocrine tumors of the gastro-entero-pancreatic system. World J Gastroenterol. 2008. 14:5377–5384.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroendocrine Tumors of the Female Reproductive Tract: A Literature Review
- Diagnosis and Treatment of Gastric Neuroendocrine Tumors
- Non-Functioning, Malignant Pancreatic Neuroendocrine Tumor in a 16-Year-old Boy: A Case Report
- Clinical and Immunohistochemical Characteristics of Pancreatic Neuroendocrine Tumor: Immunohistochemical Analysis of 7 Tumors
- Surgical Results of Pancreatic Neuroendocrine Tumors