Nat Prod Sci.
2019 Jun;25(2):143-149. 10.20307/nps.2019.25.2.143.
Enhanced Antioxidant and Anticancer Properties of Processed Eucommiae Cortex
- Affiliations
-
- 1Department of Natural Medicine Resources, Semyung University, Jecheon, Chungbuk 27136, Korea. hwalee@semyung.ac.kr
- 2School of Industrial Bio-Pharmaceutical Science, Semyung University, Jecheon, Chungbuk 27136, Korea.
- KMID: 2452784
- DOI: http://doi.org/10.20307/nps.2019.25.2.143
Abstract
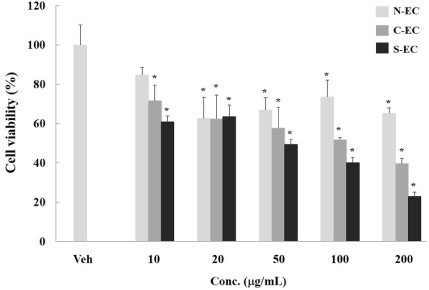
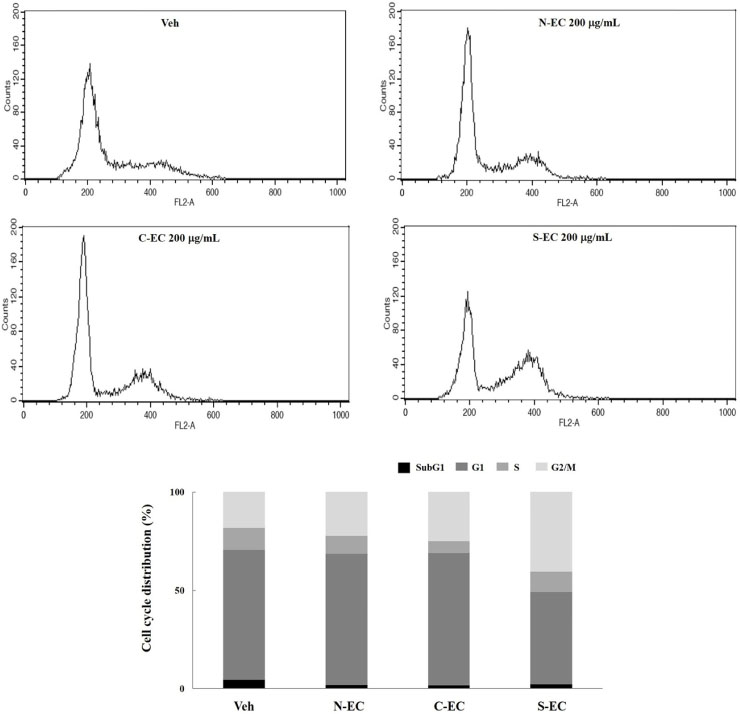
- Eucommiae Cortex (EC), bark of Eucommia ulmoides, has been known as a traditional medicine to regulate hypertension and immune system. Because silk of gum in the EC blocks the release of active ingredients, EC generally has been utilized after processing with carbonization or salt-water to breakdown it. This study aimed to investigate the differences of non-processed EC and processed EC on antioxidant and anticancer properties. Antioxidant capacity was assessed by measuring the content of total polyphenols, reducing power, and ABTS radical scavenging effect. And anticancer effects were examined by evaluating the viability of pancreatic cancer cells and wound healing ability. The results demonstrated that processed EC contained more content of polyphenols and exhibited more potent reducing power and radical scavenging effect than non-processed EC. In addition, processed EC more efficiently inhibited proliferation and migration of pancreatic cancer cells. These results suggest that processing of medicinal plants can improve the biological properties such as antioxidant or anticancer activity, which may lead to the development of herbal medicine treatment technology.
MeSH Terms
Figure
Reference
-
1. Dai X, Huang Q, Zhou B, Gong Z, Liu Z, Shi S. Food Chem. 2013; 139:563–570.2. Hsieh CL, Yen GC. Life Sci. 2000; 66:1387–1400.3. Lang C, Liu Z, Taylor HW, Baker DG. Am J Chin Med. 2005; 33:215–230.4. Choi YH, Seo JH, Kim JS, Heor JH, Kim SK, Choi SU, Kim YS, Kim YK, Ryu SY. Korean J Pharmacogn. 2003; 34:308–313.5. Kim MC, Kim DS, Kim SJ, Park J, Kim HL, Kim SY, Ahn KS, Jang HJ, Lee SG, Lee KM, Hong SH, Um JY. Am J Chin Med. 2012; 40:135–149.6. Hirata T, Kobayashi T, Wada A, Ueda T, Fujikawa T, Miyashita H, Ikeda T, Tsukamoto S, Nohara T. Bioorg Med Chem Lett. 2011; 21:1786–1791.7. Zhang R, Liu ZG, Li C, Hu SJ, Liu L, Wang JP, Mei QB. Bone. 2009; 45:553–559.8. He X, Wang J, Li M, Hao D, Yang Y, Zhang C, He R, Tao R. J Ethnopharmacol. 2014; 151:78–92.9. Chai X, Wang Y, Su Y, Bah AJ, Hu L, Gao Y, Gao X. J Pharm Biomed Anal. 2012; 57:52–61.10. Seo CS, Kim JH, Shin HK, Kim BS. Korean J Pharmacogn. 2015; 46:123–132.11. Singleton VL, Rossi JA. Am J Enol Vitic. 1965; 16:144–158.12. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Free Radic Biol Med. 1999; 26:1231–1237.13. Oyaizu M. Jpn J Nutr. 1986; 44:307–315.14. Chen XY, Luo LI, Ren GC, Qu GY, Dong LS. West China J Pharm Sci . 2008; 5:592–593.15. Tao Y, Sheng C, Li WD, Cai BC, Lu TL. Zhongguo Zhong Yao Za Zhi. 2014; 39:4352–4355.16. Yen GC, Hsieh CL. J Agric Food Chem. 1998; 46:3952–3957.17. Hu W, Wang G, Li P, Wang Y, Si CL, He J, Long W, Bai Y, Feng Z, Wang X. Chem Biol Interact. 2014; 224:108–116.18. Milkovic L, Siems W, Siems R, Zarkovic N. Curr Pharm Des. 2014; 20:6529–6542.19. Talero E, Avila-Roman J, Motilva V. Curr Pharm Des. 2012; 18:3939–3965.20. Zhu Y, Hao W, Li X. Zhongguo Zhong Yao Za Zhi. 1997; 22:598–601.21. Wells A, Grahovac J, Wheeler S, Ma B, Lauffenburger D. Trends Pharmacol Sci. 2013; 34:283–289.22. Li Y, Han C, Wang J, Xiao W, Wang Z, Zhang J, Yang Y, Zhang S, Ai C. J Ethnopharmacol. 2014; 151:452–460.23. Riaz A, Rasul A, Hussain G, Zahoor MK, Jabeen F, Subhani Z, Younis T, Ali M, Sarfraz I, Selamoglu Z. Adv Pharmacol Sci. 2018; 2018:9794625.24. Soromou LW, Chen N, Jiang L, Huo M, Wei M, Chu X, Millimouno FM, Feng H, Sidime Y, Deng X. Biochem Biophys Res Commun. 2012; 419:256–261.25. Cho IH, Gong JH, Kang MK, Lee EJ, Park JH, Park SJ, Kang YH. BMC Pulm Med. 2014; 14:122.26. Chen M, Cai F, Zha D, Wang X, Zhang W, He Y, Huang Q, Zhuang H, Hua ZC. Oncotarget. 2017; 8:26941–26958.27. Choi SY, Ko HC, Ko SY, Hwang JH, Park JG, Kang SH, Han SH, Yun SH, Kim S. J Biol Pharm Bull. 2007; 30:772–778.28. Chen QC, Zhang WY, Jin W, Lee IS, Min BS, Jung HJ, Na M, Lee S, Bae K. Planta Med. 2010; 76:79–81.29. Ganeshpurkar A, Saluja AK. Saudi Pharm J. 2017; 25:149–164.30. Lin JP, Yang JS, Lu CC, Chiang JH, Wu CL, Lin JJ, Lin HL, Yang MD, Liu KC, Chiu TH, Chung JG. Leuk Res. 2009; 33:823–828.31. Chen H, Miao Q, Geng M, Liu J, Hu Y, Tian L, Pan J, Yang Y. ScientificWorldJournal. 2013; 2013:269165.32. Araújo JR, Gonçalves P, Martel F. Nutr Res. 2011; 31:77–87.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of antioxidant and anticancer activities of fermented pericarp extract of Camellia japonica L. using Aspergillus oryzae in oral cancer
- Antioxidant activity of extracts from Euryale ferox seed
- Comparative Analysis of Anticancer and Antibacterial Activities among Seven Trametes Species
- Assessment of In vitro Antioxidant, Antidiabetic and Cytotoxic Activities of Sphaeranthus africanus Extracts
- Sorbus rufopilosa Extract Exhibits Antioxidant and Anticancer Activities by Inducing Cell Cycle Arrest and Apoptosis in Human Colon Adenocarcinoma HT29 Cells