Nat Prod Sci.
2019 Jun;25(2):122-129. 10.20307/nps.2019.25.2.122.
Quantitation and Radical Scavenging Activity Evaluation of Iridoids and Phenylethanoids from the Roots of Phlomis umbrosa (Turcz.) using DPPH Free Radical and DPPH-HPLC Methods, and their Cytotoxicity
- Affiliations
-
- 1Drug Research and Development Center, College of Pharmacy, Catholic University of Daegu, Gyeongbuk 712-702, Korea. woomh@cu.ac.kr
- 2Division of Computational Physics, Institute for Computational Science, Ton Duc Thang University, Ho Chi Minh City, Vietnam.
- 3Faculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City, Vietnam.
- 4Centre for Drug Research and Technology Transfer, Phutho College of Pharmacy, Viettri City, Phutho Province 290000, Vietnam.
- 5School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China.
- 6ChemOn Inc., Gyeonggi Bio-Research Center, Yongin, Gyeonggi 17162, Republic of Korea.
- KMID: 2452781
- DOI: http://doi.org/10.20307/nps.2019.25.2.122
Abstract
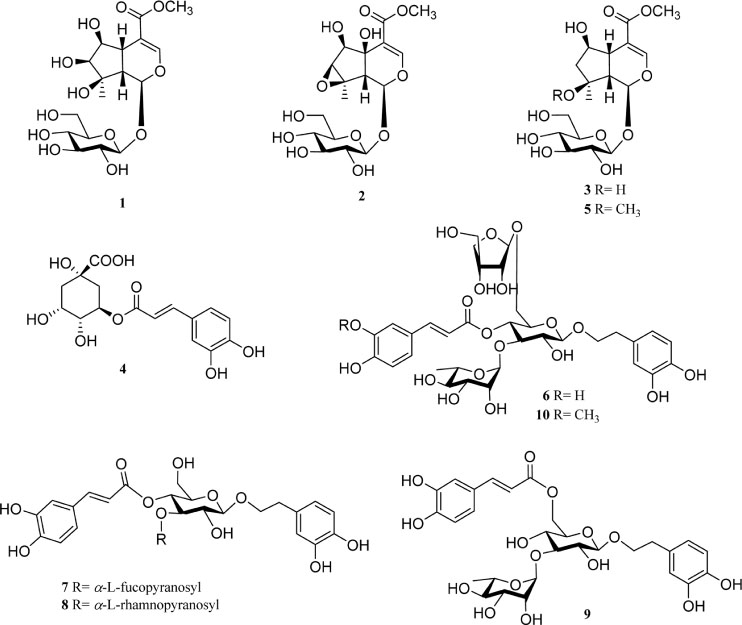
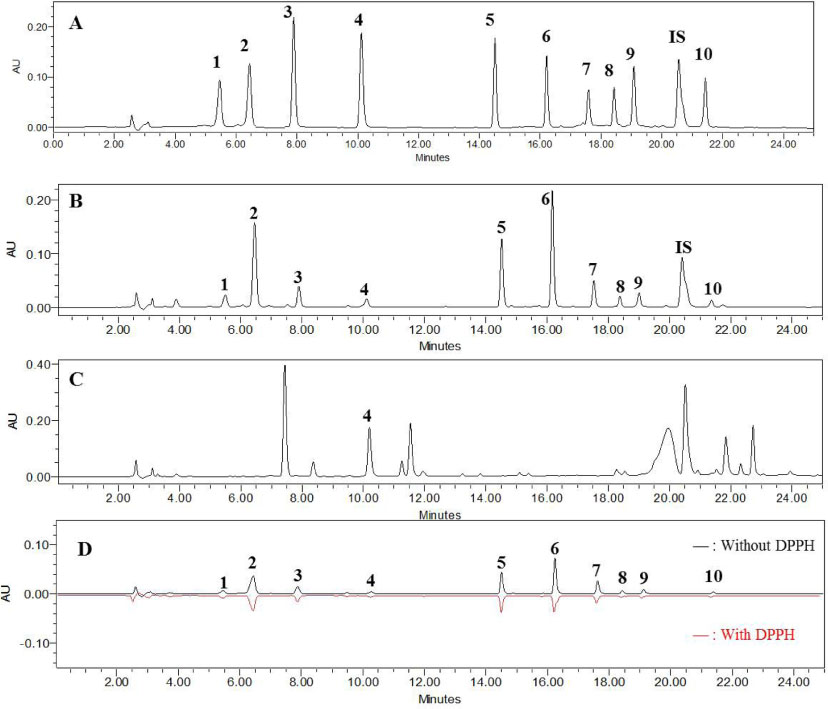
- The roots of Phlomis umbrosa (Turcz.) (Phlomidis Radix) have been traditionally used to treat cold, reduce swelling and staunch bleeding. Four iridoids (1 - 3 and 5) and six phenylethanoid derivatives (4, and 6 - 10) were isolated from the roots of P. umbrosa. A simple, sensitive, and reliable analytical HPLC/PDA method was developed, validated, and applied to determine 10 marker compounds in Phlomidis Radix. Furthermore, the isolates were evaluated for cytotoxic and anti-oxidant activities as well as DPPH-HPLC method. Among them, compounds 4 and 6 - 9 displayed potent anti-oxidant capacities using DPPH assay with IC50 values of 27.7 ± 2.4, 10.2 ± 1.1, 18.0 ± 0.8, 19.1 ± 0.3, and 19.9 ± 0.6 µM, and compounds 6, 8, and 9 displayed significant cytotoxic activity against HL-60 with IC50 values of 35.4 ± 3.1, 18.6 ± 2.0, and 42.9 ± 3.0 µM, respectively.
Figure
Reference
-
1. Andary C, Wylde R, Laffite C, Privat G, Winternitz F. Phytochemistry. 1982; 21:1123–1127.2. Zhang Y, Wang ZZ. J Pharm Biomed Anal. 2008; 47:213–217.3. Liu P, Takaishi T, Duan HQ. Chin Chem Lett. 2007; 18:155–157.4. Limem-Ben Amor I, Boubaker J, Ben Sgaier M, Skandrani I, Bhouri W, Neffati A, Kilani S, Bouhlel I, Ghedira K, Chekir-Ghedira L. J Ethnopharmacol. 2009; 125:183–202.5. Zhang Y, Wang ZZ. C R Biol. 2009; 332:816–826.6. Yun JS, Kim J, Choi J, Kwon K, Jo CH. Korean J Food Sci Technol. 2016; 48:531–535.7. Ministry of Food and Drug Safety. The Korean herbal pharmacopoeia. Korea: Shinil Books;2013. p. 250–251.8. Ministry of Food and Drug Safety. The Korean herbal pharmacopoeia. Korea: Shinil Books;2013. p. 430–431.9. Fang L, Zhang H, Zhou J, Geng Y, Wang X. J Anal Methods Chem. 2018; 2018:3158293–3158299.10. Angius F, Floris A. Toxicol In Vitro. 2015; 29:314–319.11. Kobayashi S, Mima A, Kihara M, Imakura Y. Chem Pharm Bull. 1986; 34:876–880.12. Kasai R, Katagiri M, Ohtani K, Yamasaki K, Yang CR, Tanaka O. Phytochemistry. 1994; 36:967–970.13. Clifford MN, Johnston KL, Knight S, Kuhnert N. J Agric Food Chem. 2003; 51:2900–2911.14. Saracoglu I, Kojima K, Harput US, Ogihara Y. Chem Pharm Bull. 1998; 46:726–727.15. Suo M, Ohta T, Takano F, Jin S. Molecules. 2013; 18:7336–7345.16. Li L, Tsao R, Liu Z, Liu S, Yang R, Young JC, Zhu H, Deng Z, Xie M, Fu Z. J Chromatogr A. 2005; 1063:161–169.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant Compounds Isolated from the Roots of Phlomis umbrosa Turcz.
- Quality Evaluation of Brown Rice Sulgidduk Containing Acorn Powder
- Effects of Antioxidative, DPPH Radical Scavenging Activity and Antithrombogenic by the Extract of Sancho ( Zanthoxylum Schinifolium)
- Free radical scavenging and antioxidant enzyme fortifying activities of extracts from Smilax china root
- Identification of Antioxidative Constituents from Polygonum aviculare using LC-MS Coupled with DPPH Assay