Nat Prod Sci.
2016 Mar;22(1):64-69. 10.20307/nps.2016.22.1.64.
Identification of Antioxidative Constituents from Polygonum aviculare using LC-MS Coupled with DPPH Assay
- Affiliations
-
- 1College of Pharmacy, Korea University, Sejong 339-700, Korea. kylee11@korea.ac.kr
- 2College of Pharmacy, Keimyung University, Daegu 704-701, Korea.
- KMID: 2312924
- DOI: http://doi.org/10.20307/nps.2016.22.1.64
Abstract
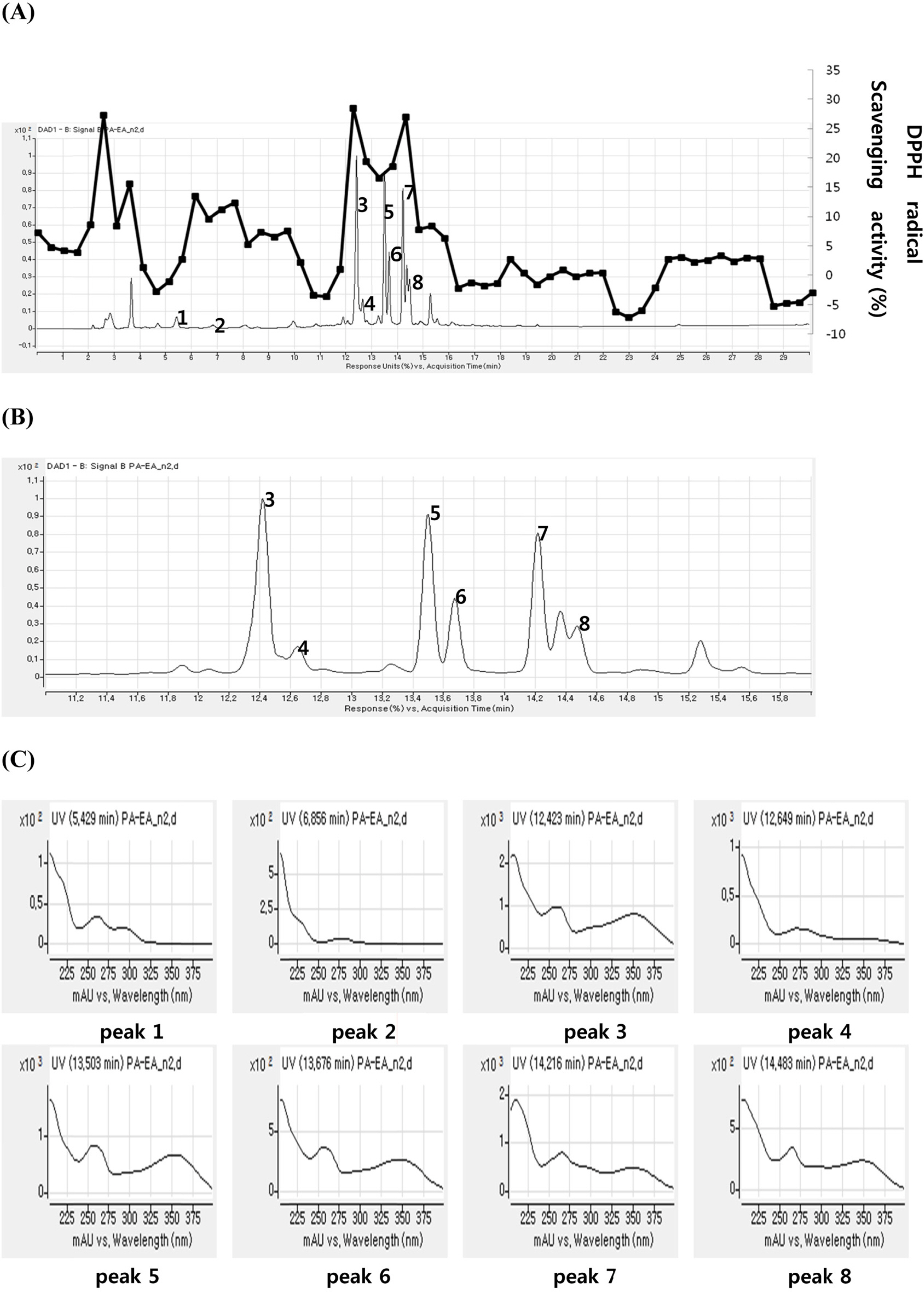
- A method for simultaneously identifying antioxidative compounds was developed using time-based LC-MS coupled with DPPH assay regardless of the time consuming process. The methanolic extract of Polygonum aviculare (Polygonaceae) showed significant DPPH radical scavenging activity. Time-based DPPH assay for simultaneous identification of active compounds from the extracts of P. aviculare was used. Major peaks of ethyl acetate fraction of P. aviculare showed high DPPH radical scavenging activity. A simple phenolic compound (1) and six flavonoids (2-7) were isolated from the ethyl acetate fraction of P. aviculare by silica gel and sephadex LH-20 column chromatography. The structures of seven compounds were determined to be protocatechuic acid (1), catechin (2), myricitrin (3), epicatechin-3-O-gallate (4), avicularin (5), quercitrin (6), and juglanin (7) based on the analysis of the 1H-NMR, 13C-NMR and ESI-MS data. All compounds exhibited significant antioxidant activity on DPPH assay and active compounds were well correlated with predicted one.
Keyword
MeSH Terms
Figure
Reference
-
(1). Qu F. N., Qi L. W., Wei Y. J., Wen X. D., Yi L., Luo H. W., Li P.Biol. Pharm. Bull. 2008; 31:501–506.(2). Nuengchamnong N., Krittasilp K., Ingkaninan K.Food Chem. 2011; 127:1287–1293.(3). Nuengchamnong N., Krittasilp K., Ingkaninan K.Food Chem. 2009; 117:750–756.(4). Nugroho A., Kim E. J., Choi J. S., Park H. J. J.Pharm. Biomed. Anal. 2014; 89:93–98.(5). Robu T., Robu B., Robu S.Environ. Eng. Manag. J. 2008; 7:579–588.(6). Granica S., Piwowarski J. P., Pop³awska M., Jakubowska M. J.Pharm. Biomed. Anal. 2013; 72:216–222.(7). Park S. H., Sung Y. Y., Nho K. J., Kim H. K. J.Ethnopharmacol. 2014; 151:1109–1115.(8). Sung Y. Y., Yoon T., Yang W. K., Kim S. J., Kim D. S., Kim H. K.Evid. Based Complement Alternat. Med. 2013; 2013:626397.(9). Hsu C. Y.Biol. Res. 2006; 39:281–288.(10). Granica S., Czerwi ska M. E., Zy y ska-Granica B., Kiss A. K.Fitoterapia. 2013; 91:180–188.(11). Nguyen P. H., Zhao B. T., Lee J. H., Kim Y. H., Min B. S., Woo M. H.Bull. Korean Chem. Soc. 2014; 35:1763–1768.(12). Seto R., Nakamura H., Nanjo F, Hara Y.Biosci. Biotech. Biochem. 1997; 61:1434–1439.(13). Aderogba M. A, Ndhlala A. R, Rengasamy K. R, Van Staden J.Molecules. 2013; 18:12633–12644.(14). Kim H. J., Woo E. R., Park H. K. J.Nat. Prod. 1994; 57:581–586.(15). Kwon D. J., Bae Y. S.Chem. Nat. Compd. 2013; 49:131–132.(16). Park B. J., Matsuta T., Kanazawa T., Park C. H., Chang K. J., Onjo M.Chem. Nat. Compd. 2012; 48:477–479.(17). Magiera S., Baranowska I., Lautenszleger A. J.Pharm. Biomed. Anal. 2015; 102:468–475.(18). de Beck P. O., Duoux M. G., Cartier G., Mariotte A. M.Phytochemistry. 1998; 47:1171–1173.(19). Sroka Z., Cisowski W.Food Chem. Toxicol. 2003; 41:753–758.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bioassay-coupled LC-QTOF MS/MS to Characterize Constituents Inhibiting Nitric Oxide Production of Thuja orientalis
- LC–MS/MS-Based Comparative Investigation on Chemical Constituents of Six Aster Species Occurring in Korea
- Phytochemical Constituents from the Rhizomes of Osmunda japonica Thunb and Their Anti-oxidant Activity
- A Sensitive and Specific Liquid ChromatographyTandem Mass Spectrometry Assay for Simultaneous Quantification of Salivary Melatonin and Cortisol: Development and Comparison With Immunoassays
- Two cases of toxic hepatitis after Polygonum multiflorum Thunb. ingestion