Nat Prod Sci.
2019 Jun;25(2):111-114. 10.20307/nps.2019.25.2.111.
Simultaneous Analysis of Four Standards of The Herbal Formula, DF-02, of Ephedra intermedia and Rheum palmatum, using by High Performance Liquid Chromatography-Ultraviolet Detector (HPLC-UVD)
- Affiliations
-
- 1College of Pharmacy, Kangwon National University, Chuncheon 24341, Korea. heejyang@kangwon.ac.kr
- 2Department of Microbiology, College of Medicine, Chung-Ang University, Seoul 06974, Korea.
- 3Department of Formula Sciences, College of Korean Medicine, Dong-Eui University, Busan 47227, Korea.
- KMID: 2452779
- DOI: http://doi.org/10.20307/nps.2019.25.2.111
Abstract
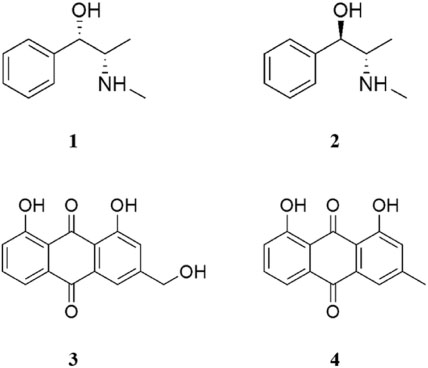
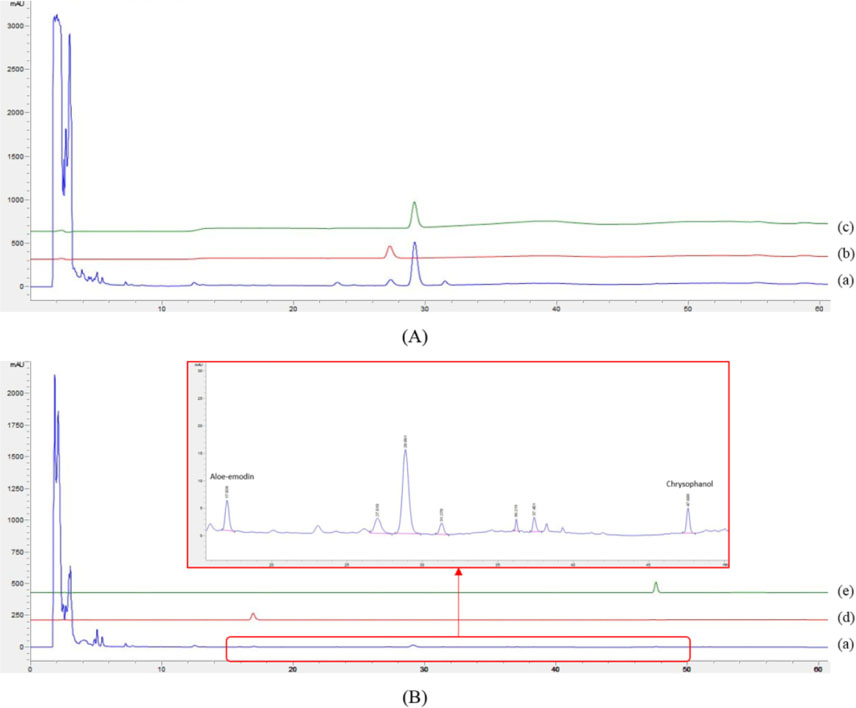
- The herbal formula, DF-02, consisting of Ephedra intermedia and Rheum palmatum are used for the treatment of the metabolic diseases such as obesity and liver fibrosis in Korean local clinics. We aimed to develop the simultaneous analytical conditions for four standards, (+)-pseudoephedrine (PSEP) and (−)-ephedrine (EP) for E. intermedia, and aloe-emodin (AE) and chrysophanol (CP) for R. palmatum using HPLC-UV techniques. The validated conditions yielded the high precision (relative standard deviation (RSD) < 3.65%) and the recoveries (94 - 106%) using the calibration curves with high linearity (R²> 0.9994). As a result, four standards of DF-02 were simultaneously determined under the developed method, which will be utilized for the quality control or evaluation of DF-02 and many herbal preparations containing E. intermedia and R. palmatum.
Keyword
MeSH Terms
Figure
Reference
-
1. Jeong B, Choi SY, Jang HS, Yoo G, Kim SH, Kim J, Kwon YS, Roh JS, Yoon Y, Shin SS. Nat Prod Sci. 2017; 23:9–15.2. Kee CH. The Pharmacology of Chinese Herbs. U.S.A.: CRC Press;1999. p. 308.3. Yamada I, Goto T, Takeuchi S, Ohshima S, Yoneyama K, Shibuya T, Kataoka E, Segawa D, Sato W, Dohmen T, Anezaki Y, Ishii H, Ohnishi H. Cytokine. 2008; 41:293–301.4. Ma G, Bavadekar SA, Davis YM, Lalchandani SG, Nagmani R, Schaneberg BT, Khan IA, Feller DR. J Pharmacol Exp Ther. 2007; 322:214–221.5. Fleming RM. Expert Opin Drug Saf. 2008; 7:749–759.
Article6. Chen DC, Wang L. Chin J Traumatol. 2009; 12:365–369.7. Barceloux DG. Dis Mon. 2009; 55:403–411.8. Sheu S, Huang M. J Food Drug Anal. 2000; 8:337–341.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of Extraction Conditions for Active Compounds of Herbal Medicinal Formula, DF, by Response Surface Methodology
- Simultaneous Determination of Four Compounds from Artemisia capillaris using High Performance Liquid Chromatography-Ultraviolet Detector (HPLC-UVD) and Their Quantitative Study in Artemisia Genus
- Quantitative Analysis and Enantiomeric Separation of Ephedra Alkaloids in Ma Huang Related Products by HPLC-DAD and UPLC-MS/MS
- Simultaneous Determination of Glutamate, Glycine, and Alanine in Human Plasma Using Precolumn Derivatization with 6-Aminoquinolyl-N-hydroxysuccinimidyl Carbamate and High-Performance Liquid Chromatography
- Development of high-performance liquid chromatography methods for the anticoccidials: toltrazuril and diclazuril