J Rheum Dis.
2019 Jul;26(3):191-199. 10.4078/jrd.2019.26.3.191.
Longitudinal Changes in the European League Against Rheumatism Sjögren's Syndrome Patient Reported Index in Real-Life Practice
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University Hospital, Cheongju, Korea.
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. yn35@snu.ac.kr
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4WCU Department of Molecular Medicine and Biopharmaceutical Sciences, Medical Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2451511
- DOI: http://doi.org/10.4078/jrd.2019.26.3.191
Abstract
OBJECTIVE
To investigate longitudinal changes in the European League Against Rheumatism (EULAR) Sjögren's syndrome patient reported index (ESSPRI) and to study the clinical features associated with favorable ESSPRI changes in primary Sjögren's syndrome (pSS).
METHODS
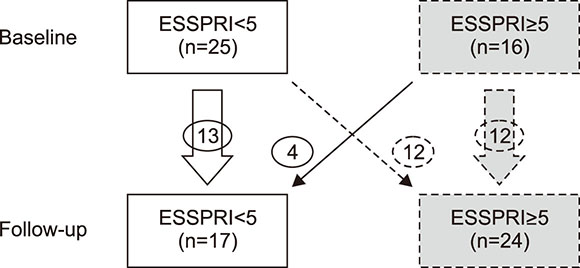
At baseline and after a median period of 6.6 years, 41 pSS patients were evaluated using the ESSPRI, EULAR Sjögren's syndrome disease activity index (ESSDAI), short-form 36, xerostomia inventory (XI), and visual analog scale (VAS) scores for symptoms. The favorable subgroup included patients who were stable or showed improved to satisfactory symptom status (ESSPRI<5) and the unfavorable subgroup included those with stable or worsening to an unsatisfactory symptom status (ESSPRI ≥5).
RESULTS
Median ESSPRI increased from 4.11 to 5.33 (p<0.05), although XI scores (p=0.01) and oral dryness (p<0.05) were significantly decreased. Serum immunoglobulin G level was significantly reduced (p<0.001) but ESSDAI scores were unchanged. Six (14.6%) patients showed clinical improvement in ESSDAI, and 11 (26.8%) showed improvement in ESSPRI. On comparing the favorable (n=17) and unfavorable (n=24) subgroups, the former exhibited significantly lower VAS scores for sicca and depression and XI and ESSPRI scores at baseline (all p<0.05) and more lacrimal flow (p<0.05). The favorable subgroup received a significantly lower cumulative dose of pilocarpine and glucocorticoids (both p<0.05).
CONCLUSION
About 25% of pSS patients showed clinically significant ESSPRI improvement and about 40% showed a favorable ESSPRI course. Because the favorable subgroup had more lacrimal flow and less sicca symptoms at baseline, long-term patient-derived outcomes could depend on residual exocrine function at pSS diagnosis.
MeSH Terms
Figure
Cited by 1 articles
-
Longitudinal Changes of the European League Against Rheumatism Sjögren's Syndrome Patient Reported Index in Korean Patients with Primary Sjögren's Syndrome
Seung-Ki Kwok
J Rheum Dis. 2019;26(4):219-220. doi: 10.4078/jrd.2019.26.4.219.
Reference
-
1. Rischmueller M, Tieu J, Lester S. Primary Sjögren's syndrome. Best Pract Res Clin Rheumatol. 2016; 30:189–220.2. Brito-Zerón P, Baldini C, Bootsma H, Bowman SJ, Jonsson R, Mariette X, et al. Sjögren syndrome. Nat Rev Dis Primers. 2016; 2:16047.3. Cornec D, Chiche L. Is primary Sjögren's syndrome an orphan disease? A critical appraisal of prevalence studies in Europe. Ann Rheum Dis. 2015; 74:e25.4. Qin B, Wang J, Yang Z, Yang M, Ma N, Huang F, et al. Epidemiology of primary Sjögren's syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015; 74:1983–1989.5. Flores-Chávez A, Kostov B, Solans R, Fraile G, Maure B, Feijoo-Massó C, et al. GEAS-SS SEMI Registry. Severe, life-threatening phenotype of primary Sjögren's syndrome: clinical characterisation and outcomes in 1580 patients (GEAS-SS Registry). Clin Exp Rheumatol. 2018; 36:Suppl 112. 121–129.6. Theander E, Manthorpe R, Jacobsson LT. Mortality and causes of death in primary Sjögren's syndrome: a prospective cohort study. Arthritis Rheum. 2004; 50:1262–1269.7. Singh AG, Singh S, Matteson EL. Rate, risk factors and causes of mortality in patients with Sjögren's syndrome: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford. . 2016; 55:450–460.8. Cho HJ, Yoo JJ, Yun CY, Kang EH, Lee HJ, Hyon JY, et al. The EULAR Sjogren's syndrome patient reported index as an independent determinant of health-related quality of life in primary Sjogren's syndrome patients: in comparison with non-Sjogren's sicca patients. Rheumatology (Oxford. . 2013; 52:2208–2217.9. Strömbeck B, Ekdahl C, Manthorpe R, Wikström I, Jacobsson L. Health-related quality of life in primary Sjögren's syndrome, rheumatoid arthritis and fibromyalgia compared to normal population data using SF-36. Scand J Rheumatol. 2000; 29:20–28.10. Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, et al. Primary Sjögren's syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009; 7:46.11. Seror R, Ravaud P, Mariette X, Bootsma H, Theander E, Hansen A, et al. EULAR Sjögren's Task Force. EULAR Sjogren's Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjogren's syndrome. Ann Rheum Dis. 2011; 70:968–972.12. Seror R, Theander E, Bootsma H, Bowman SJ, Tzioufas A, Gottenberg JE, et al. Outcome measures for primary Sjögren's syndrome: a comprehensive review. J Autoimmun. 2014; 51:51–56.13. Hackett KL, Newton JL, Frith J, Elliott C, Lendrem D, Foggo H, et al. Impaired functional status in primary Sjögren's syndrome. Arthritis Care Res (Hoboken. . 2012; 64:1760–1764.14. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–558.15. Seror R, Bowman SJ, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, et al. EULAR Sjögren's syndrome disease activity index (ESSDAI): a user guide. RMD Open. 2015; 1:e000022.16. Seror R, Bootsma H, Saraux A, Bowman SJ, Theander E, Brun JG, et al. EULAR Sjögren's Task Force. Defining disease activity states and clinically meaningful improvement in primary Sjögren's syndrome with EULAR primary Sjögren's syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann Rheum Dis. 2016; 75:382–389.17. Gannot G, Lancaster HE, Fox PC. Clinical course of primary Sjögren's syndrome: salivary, oral, and serologic aspects. J Rheumatol. 2000; 27:1905–1909.18. Pertovaara M, Pukkala E, Laippala P, Miettinen A, Pasternack A. A longitudinal cohort study of Finnish patients with primary Sjögren's syndrome: clinical, immunological, and epidemiological aspects. Ann Rheum Dis. 2001; 60:467–472.19. Theander E, Andersson SI, Manthorpe R, Jacobsson LT. Proposed core set of outcome measures in patients with primary Sjögren's syndrome: 5 year follow up. J Rheumatol. 2005; 32:1495–1502.20. Haldorsen K, Bjelland I, Bolstad AI, Jonsson R, Brun JG. A five-year prospective study of fatigue in primary Sjögren's syndrome. Arthritis Res Ther. 2011; 13:R167.21. Gazeau P, Cornec D, Jousse-Joulin S, Guellec D, Saraux A, Devauchelle-Pensec V. Time-course of ultrasound abnormalities of major salivary glands in suspected Sjögren's syndrome. Joint Bone Spine. 2018; 85:227–232.22. Kruize AA, Hené RJ, Kallenberg CG, vanBijsterveld OP, vander Heide A, Kater L, et al. Hydroxychloroquine treatment for primary Sjögren's syndrome: a two year double blind crossover trial. Ann Rheum Dis. 1993; 52:360–364.23. Miyawaki S, Nishiyama S, Matoba K. Efficacy of low-dose prednisolone maintenance for saliva production and serological abnormalities in patients with primary Sjögren's syndrome. Intern Med. 1999; 38:938–943.24. Nakayamada S, Saito K, Nakatsuka K, Nakano K, Tokunaga M, Sawamukai N, et al. Efficacy of mizoribine treatment in patients with Sjögren's syndrome: an open pilot trial. Mod Rheumatol. 2003; 13:339–345.25. Liang Y, Yang Z, Qin B, Zhong R. Primary Sjogren's syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis. 2014; 73:1151–1156.26. Rúa-Figueroa I, Fernández Castro M, Andreu JL, Sanchez-Piedra C, Martínez-Taboada V, Olivé A, et al. Sjogrenser and Relesser Researchers and EAS-SER Group. Comorbidities in patients with primary Sjögren's syndrome and systemic lupus erythematosus: a comparative registries-based study. Arthritis Care Res (Hoboken). 2017; 69:38–45.27. Haldorsen K, Moen K, Jacobsen H, Jonsson R, Brun JG. Exocrine function in primary Sjögren syndrome: natural course and prognostic factors. Ann Rheum Dis. 2008; 67:949–954.28. Johnsen SJ, Brun JG, Gøransson LG, Småstuen MC, Johannesen TB, Haldorsen K, et al. Risk of non-Hodgkin's lymphoma in primary Sjögren's syndrome: a populationbased study. Arthritis Care Res (Hoboken. . 2013; 65:816–821.29. Nishishinya MB, Pereda CA, Muñoz-Fernández S, Pego-Reigosa JM, Rúa-Figueroa I, Andreu JL, et al. Identification of lymphoma predictors in patients with primary Sjögren's syndrome: a systematic literature review and meta-analysis. Rheumatol Int. 2015; 35:17–26.30. Omma A, Tecer D, Kucuksahin O, Sandikci SC, Yildiz F, Erten S. Do the European League Against Rheumatism (EULAR) Sjögren's syndrome outcome measures correlate with impaired quality of life, fatigue, anxiety and depression in primary Sjögren's syndrome. Arch Med Sci. 2018; 14:830–837.31. Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, DelPino-Montes J, et al. GEMESS Study Group. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore). 2008; 87:210–219.32. García-Carrasco M, Sisó A, Ramos-Casals M, Rosas J, delaRed G, Gil V, et al. Raynaud's phenomenon in primary Sjögren's syndrome. Prevalence and clinical characteristics in a series of 320 patients. J Rheumatol. 2002; 29:726–730.33. Tishler M, Yaron I, Shirazi I, Yaron M. Clinical and immunological characteristics of elderly onset Sjögren's syndrome: a comparison with younger onset disease. J Rheumatol. 2001; 28:795–797.34. Botsios C, Furlan A, Ostuni P, Sfriso P, Andretta M, Ometto F, et al. Elderly onset of primary Sjögren's syndrome: clinical manifestations, serological features and oral/ocular diagnostic tests. Comparison with adult and young onset of the disease in a cohort of 336 Italian patients. Joint Bone Spine. 2011; 78:171–174.35. Mignogna MD, Fedele S, LoRusso L, LoMuzio L, Wolff A. Sjögren's syndrome: the diagnostic potential of early oral manifestations preceding hyposalivation/xerostomia. J Oral Pathol Med. 2005; 34:1–6.36. Theander E, Jonsson R, Sjöström B, Brokstad K, Olsson P, Henriksson G. Prediction of Sjögren's syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol. 2015; 67:2427–2436.37. Gottenberg JE, Seror R, Miceli-Richard C, Benessiano J, Devauchelle-Pensec V, Dieude P, et al. Serum levels of beta2-microglobulin and free light chains of immunoglobulins are associated with systemic disease activity in primary Sjögren's syndrome. Data at enrollment in the prospective ASSESS cohort. PLoS One. 2013; 8:e59868.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Longitudinal Changes of the European League Against Rheumatism Sjögren's Syndrome Patient Reported Index in Korean Patients with Primary Sjögren's Syndrome
- New Classification Criteria for Primary Sjögren's Syndrome and Salivary Gland Ultrasonography
- A Case of Treatment with Steroid and Hydrochloroquine of Thrombocytopenia in Primary Sjögren's Syndrome
- A Case of Sjögren-Larsson Syndrome
- Rehabilitation using twin-stage method for a Sjögren's syndrome patient with severe discoloration and attrition on upper and lower anterior teeth