J Clin Neurol.
2019 Jul;15(3):328-333. 10.3988/jcn.2019.15.3.328.
Therapeutic Outcome of Alemtuzumab in Korean Patients with Multiple Sclerosis: 2-Year Follow-Up
- Affiliations
-
- 1Department of Neurology, Research Institute and Hospital of National Cancer Center, Goyang, Korea. hojinkim@ncc.re.kr
- KMID: 2451116
- DOI: http://doi.org/10.3988/jcn.2019.15.3.328
Abstract
- BACKGROUND AND PURPOSE
Alemtuzumab has shown high efficacy in clinical trials that primarily involved Western multiple sclerosis (MS) patients. To evaluate the therapeutic outcome of alemtuzumab in Korean patients with MS.
METHODS
This study enrolled 23 consecutive patients who were treated with alemtuzumab from 2015 to 2018. Efficacy was evaluated using the annualized relapse rate (ARR), Expanded Disability Status Scale (EDSS), and radiological activity. No evidence of disease activity (NEDA) was defined as no clinical relapse, no worsening of the EDSS score, and no radiological activity. The safety profiles were also assessed.
RESULTS
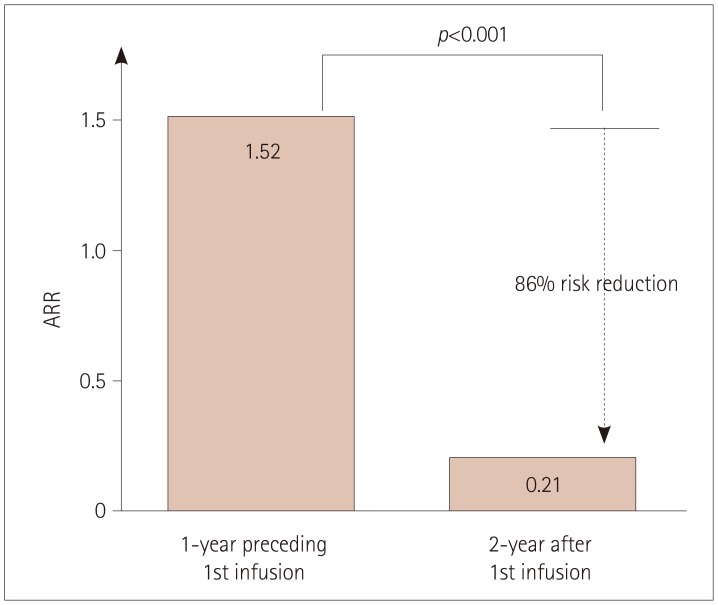
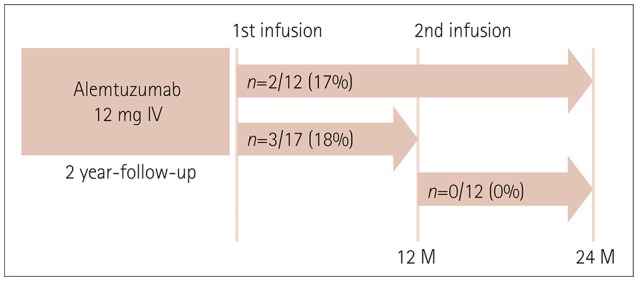
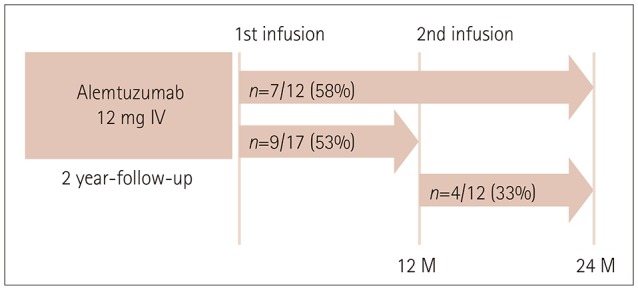
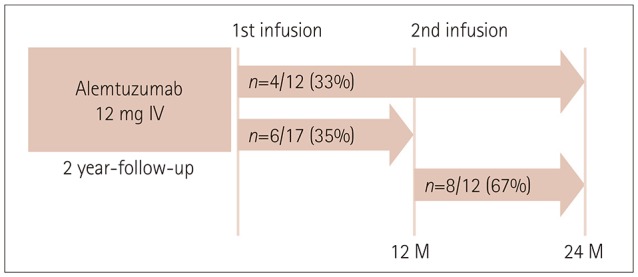
The mean age was 36 years and 16 of the patients were female. Seventeen and 12 of 23 patients were followed up for 1 year and 2 years, respectively. The ARR was markedly reduced from 1.52 during the 1-year period preceding alemtuzumab administration to 0.21 after initiating alemtuzumab (p<0.001). During the first and second years after initiating alemtuzumab, EDSS worsening was observed in 3 (18%) and 0 (0%) patients, respectively, and radiological activity was exhibited in 9 (53%) and 4 (33%). NEDA was observed in 6 (35%) patients during the first year and in 8 (67%) patients during the second year. Intriguingly, one patient experienced 2 severe clinical exacerbations, which occurred at 10 months after the first and 10 months after the second infusion of alemtuzumab. Nineteen of the 23 patients exhibited infusion-associated reactions and 3 patients exhibited herpes zoster infection. Thyroid dysfunction occurred in two patients at 18 and 20 months after initiating alemtuzumab.
CONCLUSIONS
Consistent with observations in Western populations, alemtuzumab therapy in Korean MS patients led to marked reductions of disease activity without unexpected safety issues.
Keyword
Figure
Reference
-
1. Cox AL, Thompson SA, Jones JL, Robertson VH, Hale G, Waldmann H, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005; 35:3332–3342. PMID: 16231285.
Article2. CAMMS223 Trial Investigators. Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008; 359:1786–1801. PMID: 18946064.
Article3. Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012; 380:1819–1828. PMID: 23122652.
Article4. Havrdova E, Arnold DL, Cohen JA, Hartung HP, Fox EJ, Giovannoni G, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017; 89:1107–1116. PMID: 28835401.
Article5. Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012; 380:1829–1839. PMID: 23122650.
Article6. Coles AJ, Cohen JA, Fox EJ, Giovannoni G, Hartung HP, Havrdova E, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology. 2017; 89:1117–1126. PMID: 28835403.7. Le Page E, Deburghgraeve V, Lester MA, Cardiet I, Leray E, Edan G. Alemtuzumab as rescue therapy in a cohort of 16 aggressive multiple sclerosis patients previously treated by Mitoxantrone: an observational study. J Neurol. 2015; 262:1024–1034. PMID: 25701008.
Article8. Huhn K, Bayas A, Doerck S, Frank B, Gerbershagen K, Hellwig K, et al. Alemtuzumab as rescue therapy in a cohort of 50 relapsing-remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol. 2018; 265:1521–1527. PMID: 29696498.
Article9. Prosperini L, Annovazzi P, Boffa L, Buscarinu MC, Gallo A, Matta M, et al. No evidence of disease activity (NEDA-3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36-month real-world study. J Neurol. 2018; 265:2851–2860. PMID: 30259178.
Article10. Tuohy O, Costelloe L, Hill-Cawthorne G, Bjornson I, Harding K, Robertson N, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry. 2015; 86:208–215. PMID: 24849515.
Article11. Willis MD, Harding KE, Pickersgill TP, Wardle M, Pearson OR, Scolding NJ, et al. Alemtuzumab for multiple sclerosis: long term follow-up in a multi-centre cohort. Mult Scler. 2016; 22:1215–1223. PMID: 26514979.
Article12. Kim NH, Kim HJ, Cheong HK, Kim BJ, Lee KH, Kim EH, et al. Prevalence of multiple sclerosis in Korea. Neurology. 2010; 75:1432–1438. PMID: 20956788.
Article13. Kim SH, Park MS, Kim W, Huh SY, Shin HJ, Hyun JW, et al. Real-world effectiveness of disease modifying therapies in Korean patients with relapsing multiple sclerosis. J Clin Neurol. 2019; 15:20–26. PMID: 30375760.14. Hartung HP, Aktas O, Boyko AN. Alemtuzumab: a new therapy for active relapsing-remitting multiple sclerosis. Mult Scler. 2015; 21:22–34. PMID: 25344374.
Article15. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69:292–302. PMID: 21387374.
Article16. Hyun JW, Kim Y, Kim G, Kim SH, Kim HJ. Severe B cell-mediated disease activation despite two cycles of alemtuzumab in a patient with multiple sclerosis. Mult Scler. 2018; 11. 07. [Epub]. DOI: 10.1177/1352458518810261.
Article17. Prosperini L, Gallo V, Petsas N, Borriello G, Pozzilli C. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol. 2009; 16:1202–1209. PMID: 19538207.
Article18. Rudick RA, Polman CH. Current approaches to the identification and management of breakthrough disease in patients with multiple sclerosis. Lancet Neurol. 2009; 8:545–559. PMID: 19446274.
Article19. Río J, Nos C, Tintoré M, Téllez N, Galán I, Pelayo R, et al. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol. 2006; 59:344–352. PMID: 16437558.20. Río J, Tintoré M, Sastre-Garriga J, Nos C, Castilló J, Tur C, et al. Change in the clinical activity of multiple sclerosis after treatment switch for suboptimal response. Eur J Neurol. 2012; 19:899–904. PMID: 22289050.
Article21. Kalincik T, Horakova D, Spelman T, Jokubaitis V, Trojano M, Lugaresi A, et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol. 2015; 77:425–435. PMID: 25546031.
Article22. Wade BJ. Spatial analysis of global prevalence of multiple sclerosis suggests need for an updated prevalence scale. Mult Scler Int. 2014; 2014:124578. PMID: 24693432.
Article23. Traboulsee A, Bass AD, Boster A, Berkovich R, Comi G, Fernández Ó, et al. Additional courses of alemtuzumab improved clinical and MRI outcomes in pooled CARE-MS I and II patients with disease activity after two courses: analysis of patients who received ≥3 courses. Mult Scler. 2018; 24:517–518.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined spinal-epidural anesthesia for caesarian section in a patients with multiple sclerosis: A case report
- A Case of Multiple Sclerosis in Child Showing Homonymous Hemianopia
- A Case of Unilateral Renal Angiomyolipoma Associated with Tuberous Sclerosis
- Balo's Concentric Sclerosis Mimicking Cerebral Tuberculoma
- A Case of Multiple Sclerosis