Yonsei Med J.
2015 May;56(3):598-607. 10.3349/ymj.2015.56.3.598.
Expression of Sarcosine Metabolism-Related Proteins in Invasive Lobular Carcinoma: Comparison to Invasive Ductal Carcinoma
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. kjs1976@yuhs.ac
- KMID: 2450331
- DOI: http://doi.org/10.3349/ymj.2015.56.3.598
Abstract
- PURPOSE
The aims of this study were to compare the expression of sarcosine metabolism-related proteins between invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC) and to determine the implications of these results.
MATERIALS AND METHODS
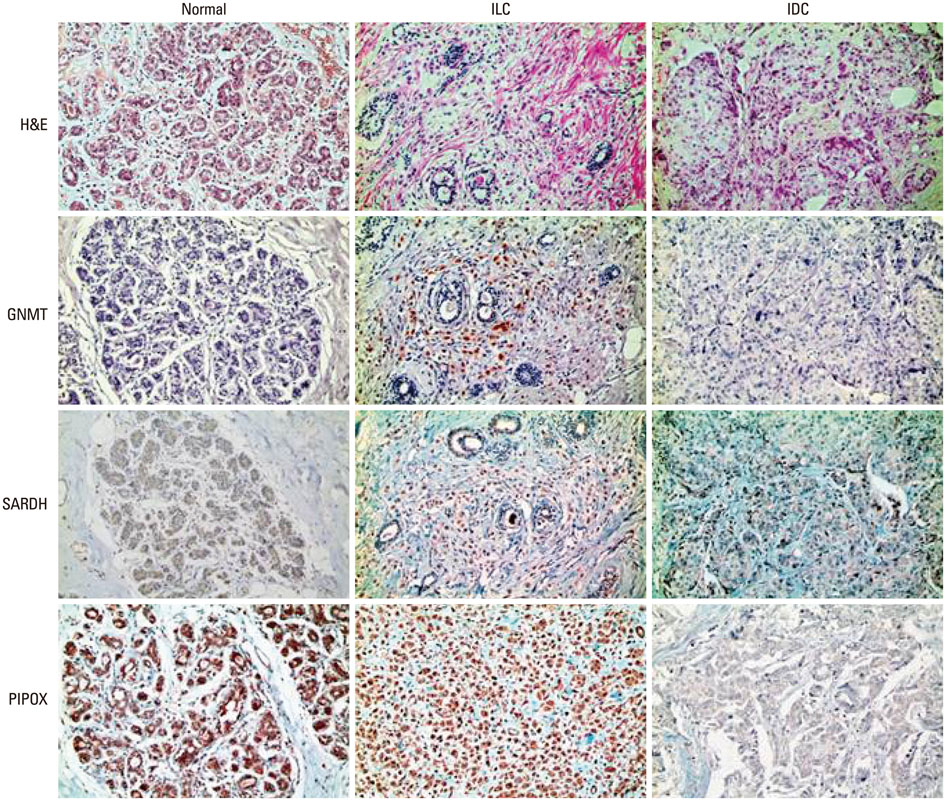
Tissue microarrays were constructed, containing 30 samples from normal breast tissue, 114 samples from patients with ILC, and 692 samples from patients with IDC. Immunohistochemical staining was performed to examine the expression of sarcosine metabolism-related proteins [glycine N-methyltransferase, sarcosine dehydrogenase, and l-pipecolic acid oxidase (PIPOX)].
RESULTS
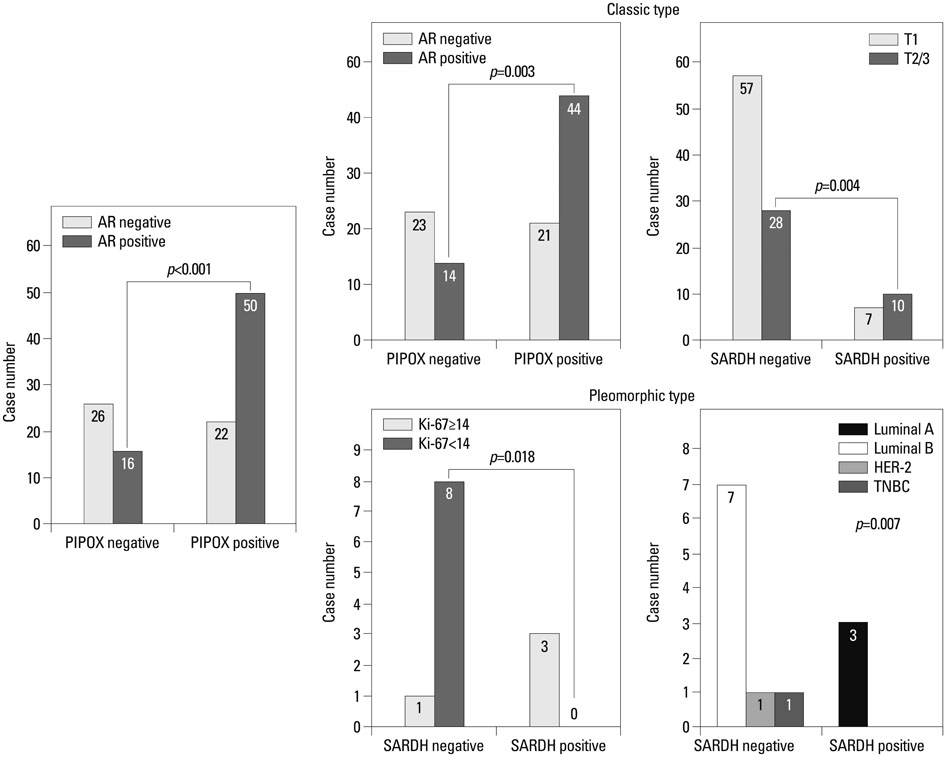
The sarcosine metabolic phenotype differed between ILC and IDC (p<0.001). In IDC, sarcosine metabolic phenotype was distributed as null type (61.7%)>low sarcosine type (30.4%)>high sarcosine type (5.0%)>intermediate type (2.9%). However, in ILC, the sarcosine metabolic phenotype was distributed as low sarcosine type (61.4%)>null type (32.5%)>intermediate type (5.3%)>high sarcosine type (0.9%). PIPOX showed higher expression in ILC than in IDC (p<0.001) and correlated with androgen receptor (AR) positivity (p=0.001) in ILC.
CONCLUSION
Expression of sarcosine metabolism-related proteins differed between ILC and IDC. Low sarcosine type was the majority sarcosine metabolic phenotype of ILC. PIPOX expression was predominant in ILC and correlated with AR positivity.
Keyword
MeSH Terms
-
Adult
Breast/pathology
Breast Neoplasms/*metabolism/pathology
Carcinoma, Ductal, Breast/*metabolism/pathology
Carcinoma, Lobular/*metabolism
Female
Humans
Immunohistochemistry
Middle Aged
Multivariate Analysis
Phenotype
Proportional Hazards Models
Regression Analysis
Retrospective Studies
Sarcosine/genetics/*metabolism
Tissue Array Analysis
Sarcosine
Figure
Cited by 1 articles
-
Detection of Circulating Tumor Cells in Breast Cancer Patients Using Cytokeratin-19 Real-Time RT-PCR
Hyung Seok Park, Hyun Ju Han, Soohyeon Lee, Gun Min Kim, Seho Park, Yeon A Choi, Jeong Dong Lee, Gi Moon Kim, Joohyuk Sohn, Seung Il Kim
Yonsei Med J. 2017;58(1):19-26. doi: 10.3349/ymj.2017.58.1.19.
Reference
-
1. Tavassoli FA, Devilee P. International Agency for Research on Cancer, World Health Organization. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IAPS Press;2003.2. Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003; 289:1421–1424.
Article3. Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005; 93:1046–1052.
Article4. Li CI, Chlebowski RT, Freiberg M, Johnson KC, Kuller L, Lane D, et al. Alcohol consumption and risk of postmenopausal breast cancer by subtype: the women's health initiative observational study. J Natl Cancer Inst. 2010; 102:1422–1431.
Article5. Reeves GK, Beral V, Green J, Gathani T, Bull D. Million Women Study Collaborators. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol. 2006; 7:910–918.
Article6. Lesser ML, Rosen PP, Kinne DW. Multicentricity and bilaterality in invasive breast carcinoma. Surgery. 1982; 91:234–240.7. Silverstein MJ, Lewinsky BS, Waisman JR, Gierson ED, Colburn WJ, Senofsky GM, et al. Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer. 1994; 73:1673–1677.
Article8. De Leeuw WJ, Berx G, Vos CB, Peterse JL, Van de Vijver MJ, Litvinov S, et al. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997; 183:404–411.
Article9. Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996; 77:113–120.
Article10. Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991; 48:28–33.
Article11. Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009; 457:910–914.
Article12. Khan AP, Rajendiran TM, Ateeq B, Asangani IA, Athanikar JN, Yocum AK, et al. The role of sarcosine metabolism in prostate cancer progression. Neoplasia. 2013; 15:491–501.
Article13. Baum CE, Price DK, Figg WD. Sarcosine as a potential prostate cancer biomarker and therapeutic target. Cancer Biol Ther. 2010; 9:341–342.
Article14. Riva C, Dainese E, Caprara G, Rocca PC, Massarelli G, Tot T, et al. Immunohistochemical study of androgen receptors in breast carcinoma. Evidence of their frequent expression in lobular carcinoma. Virchows Arch. 2005; 447:695–700.
Article15. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.
Article16. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795.
Article17. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25:118–145.
Article18. Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010; 41:107–112.
Article19. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747.
Article20. Jentzmik F, Stephan C, Lein M, Miller K, Kamlage B, Bethan B, et al. Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression. J Urol. 2011; 185:706–711.
Article21. Lee CM, Yen CH, Tzeng TY, Huang YZ, Chou KH, Chang TJ, et al. Androgen response element of the glycine N-methyltransferase gene is located in the coding region of its first exon. Biosci Rep. 2013; 33:pii: e00070.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Invasive Lobular Carcinoma of the Breast Associated with Mixed Lobular and Ductal Carcinoma In Situ: A Case Report
- Nodular Metastatic Carcinoma from Invasive Lobular Breast Cancer
- Clinicopathlogic and Immunohistochemical Characteristics of Triple Negative Invasive Lobular Carcinoma
- Uncoupling Protein 2 (UCP2) and p53 Expression in Invasive Ductal Carcinoma of Breast
- Clinical Analysis of an Invasive Lobular Carcinoma in the Breast