J Gastric Cancer.
2019 Jun;19(2):173-182. 10.5230/jgc.2019.19.e14.
Rapid Staining Using the Shorr Method for Intraoperative Peritoneal Washing Cytology in Advanced Gastric Cancer: a Pilot Study from a Single Institution
- Affiliations
-
- 1Department of Surgery, Ajou University Hospital, Suwon, Korea.
- 2Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea. hye2@snu.ac.kr
- 3Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea. hhkim@snubh.org
- KMID: 2449938
- DOI: http://doi.org/10.5230/jgc.2019.19.e14
Abstract
- PURPOSE
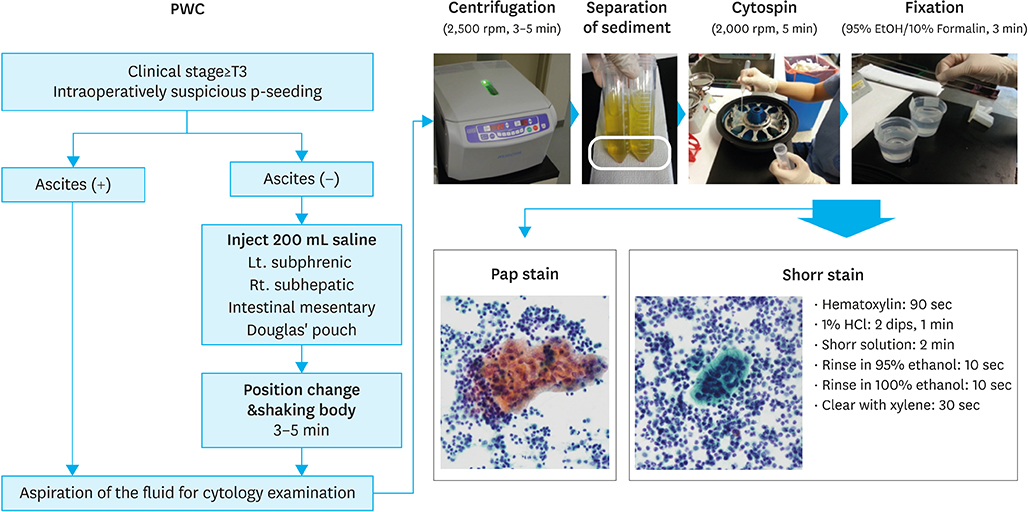
Intraoperative peritoneal washing cytology (PWC) is used to determine treatment strategies for gastric cancer with suspected serosal invasion. However, a standard staining method for intraoperative PWC remains to be established. We evaluated the feasibility of a rapid and simple staining method using Shorr's stain for intraoperative PWC in advanced gastric cancer.
MATERIALS AND METHODS
Between November 2012 and December 2014, 77 patients with clinical T3 or higher gastric cancer were enrolled. The sensitivity, specificity, and concordance between the Shorr staining method and conventional Papanicolaou (Pap) staining with carcinoembryonic antigen (CEA) immunohistochemistry (IHC) were analyzed.
RESULTS
Intraoperative PWC was performed laparoscopically in 69 patients (89.6%). The average time of the procedure was 8.3 minutes, and the average amount of aspirated fluids was 83.3 mL. The average time for Shorr staining and pathologic review was 21.0 minutes. Of the 77 patients, 16 (20.7%) had positive cytology and 7 (9.1%) showed atypical findings; sensitivity and specificity were 73.6% and 98.2% for the Shorr method, and 78.9% and 98.2% for the Pap method with CEA IHC, respectively. Concordance of diagnosis between the 2 methods was observed in 90.9% of cases (weighted κ statistic=0.875) and most disagreements in diagnoses occurred in atypical findings (6/7). In overall survival, there was no significant difference in C-index between the 2 methods (0.459 in Shorr method vs. 0.458 in Pap with CEA IHC method, P=0.987).
CONCLUSIONS
Shorr staining could be a rapid and reliable method for intraoperative PWC in advanced gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017; 3:524–548.2. Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009; 80:173–181.
Article3. Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association nationwide survey on gastric cancer in 2014. J Gastric Cancer. 2016; 6:131–140.4. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321.
Article5. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–4393.
Article6. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.7. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, editors. AJCC Cancer Staging Manual. 8th ed. New York (NY): Springer;2017.8. Jamel S, Markar SR, Malietzis G, Acharya A, Athanasiou T, Hanna GB. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer. 2018; 21:10–18.
Article9. Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg. 2016; 20:367–373.
Article10. Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013; 119:3354–3358.
Article11. Shorr E. A new technic for staining vaginal smears: iii, a single differential stain. Science. 1941; 94:545–546.
Article12. Kano Y, Kosugi S, Ishikawa T, Otani T, Muneoka Y, Sato Y, et al. Prognostic significance of peritoneal lavage cytology at three cavities in patients with gastric cancer. Surgery. 2015; 158:1581–1589.13. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016; 17:309–318.
Article14. Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009; 250:242–246.
Article15. Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017; 20:128–134.
Article16. Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014; 21:553–559.
Article17. Wong SC, Yu H, So JB. Detection of telomerase activity in gastric lavage fluid: a novel method to detect gastric cancer. J Surg Res. 2006; 131:252–255.18. Chen L, Zhang K. Surgical treatment for metastatic gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2017; 20:731–734.19. Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994; 14:2131–2134.20. Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999; 72:60–64.
Article21. Suzuki T, Ochiai T, Hayashi H, Nakajima K, Yasumoto A, Hishikawa E, et al. Importance of positive peritoneal lavage cytology findings in the stage grouping of gastric cancer. Surg Today. 1999; 29:111–115.
Article22. Burke EC, Karpeh MS Jr, Conlon KC, Brennan MF. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol. 1998; 5:411–415.
Article23. Jung M, Jeung HC, Lee SS, Park JY, Hong S, Lee SH, et al. The clinical significance of ascitic fluid CEA in advanced gastric cancer with ascites. J Cancer Res Clin Oncol. 2010; 136:517–526.
Article24. Papanicolaou GN. A new procedure for staining vaginal smears. Science. 1942; 95:438–439.
Article25. Henkel R, Schreiber G, Sturmhoefel A, Hipler UC, Zermann DH, Menkveld R. Comparison of three staining methods for the morphological evaluation of human spermatozoa. Fertil Steril. 2008; 89:449–455.
Article26. Sakuma T, Mimura A, Tanigawa N, Takamizu R, Morishima H, Matsunami N. Rapid on-site cytologic examination of 1500 breast lesions using the modified Shorr's stain. Breast Cancer. 2015; 22:280–286.
Article27. Sakuma T, Iseki R, Mimura A, Tanigawa N, Takamizu R. Rapid cytologic diagnosis of choroidal malignant melanoma by vitreous smear. J Fr Ophtalmol. 2012; 35:535.e1–535.e4.
Article28. Storti-Filho A, Estivalet Svidizinski TI, da Silva Souza RJ, de Mello IC, da Costa Souza P, Lopes Consolaro ME. Oncotic colpocytology stained with Harris-Shorr in the observation of vaginal microorganisms. Diagn Cytopathol. 2008; 36:358–362.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Shorr Versus Modified Ultrafast Papanicolaou Method for Intraoperative Diagnosis of Peritoneal Washing Cytology in Advanced Gastric Cancer: A Phase II Study
- Prognostic Value of CEA and CA19 - 9 in Serum and Peritoneal Washing Fluid in Gastric Carcinoma
- Conventional Cytology Is Not Beneficial for Predicting Peritoneal Recurrence after Curative Surgery for Gastric Cancer: Results of a Prospective Clinical Study
- Free Cancer Cell Detection in Peritoneal Cavitr of Gastric Cancer Patients by RT-PCR for CEA
- Clinical Significance of Measuring Levels of CEA, CA19-9 in Peritoneal Washing Fluid in Patients with Gastric Cancer