J Pathol Transl Med.
2019 May;53(3):192-197. 10.4132/jptm.2019.03.20.
Frozen Cytology of Meningeal Malignant Solitary Fibrous Tumor/Hemangiopericytoma
- Affiliations
-
- 1Department of Pathology Gil Medical Center, Gachon University College of Medicine, Incheon, Korea. clara_nrk@gilhospital.com
- 2Department of Neurosurgery, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- KMID: 2449343
- DOI: http://doi.org/10.4132/jptm.2019.03.20
Abstract
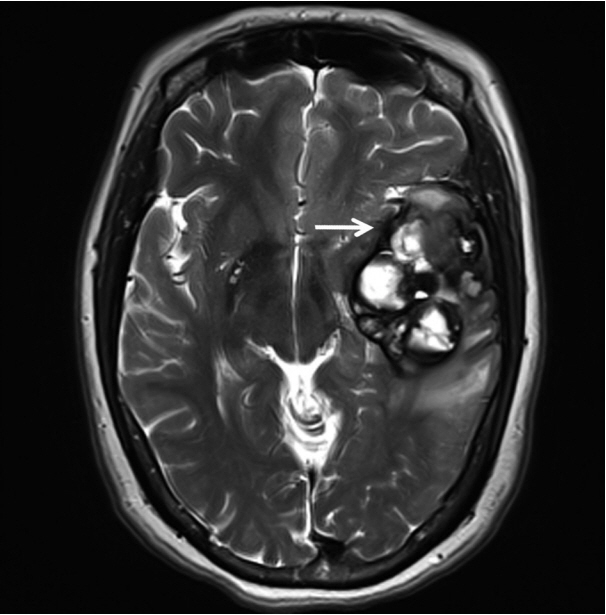
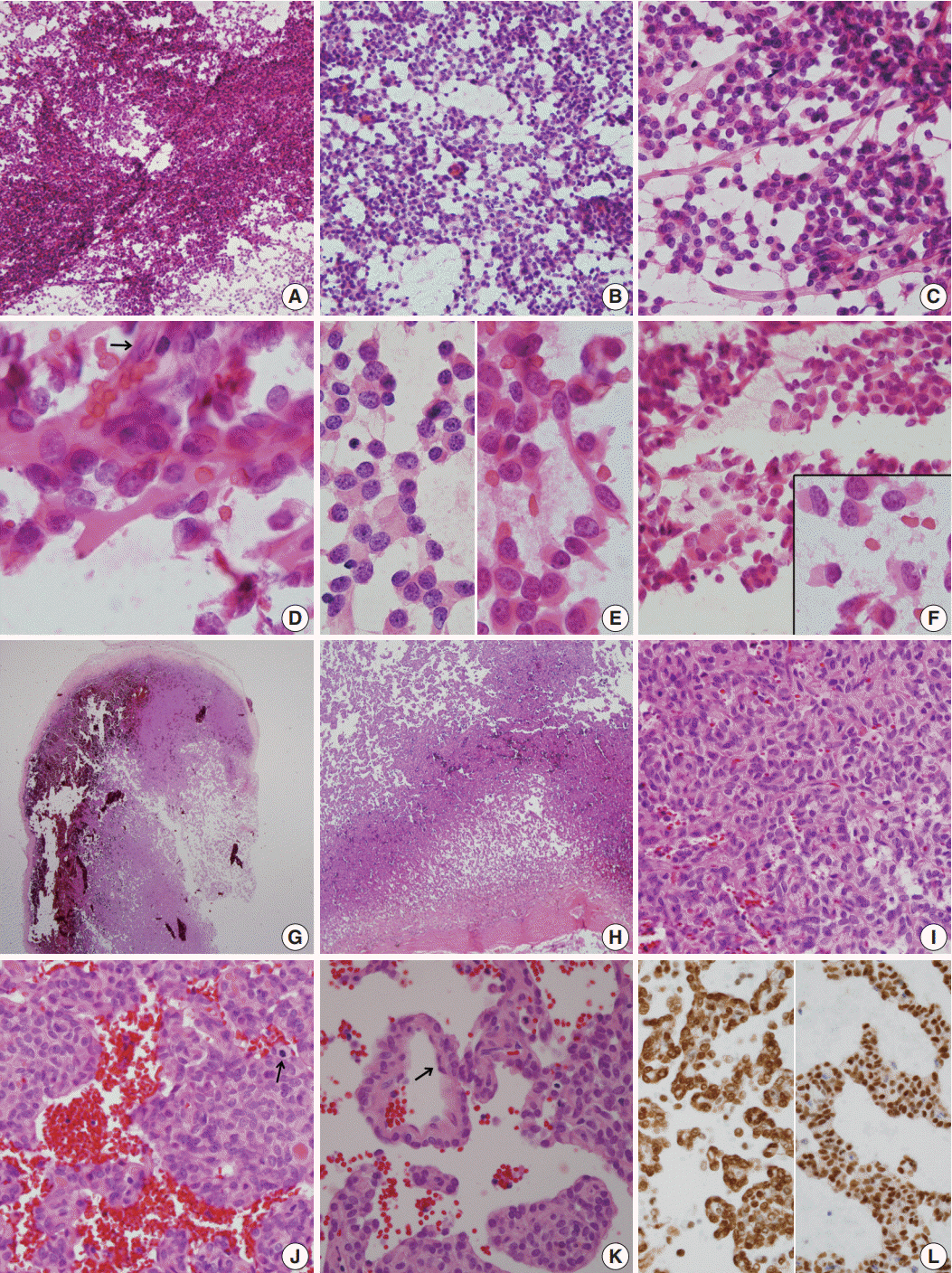
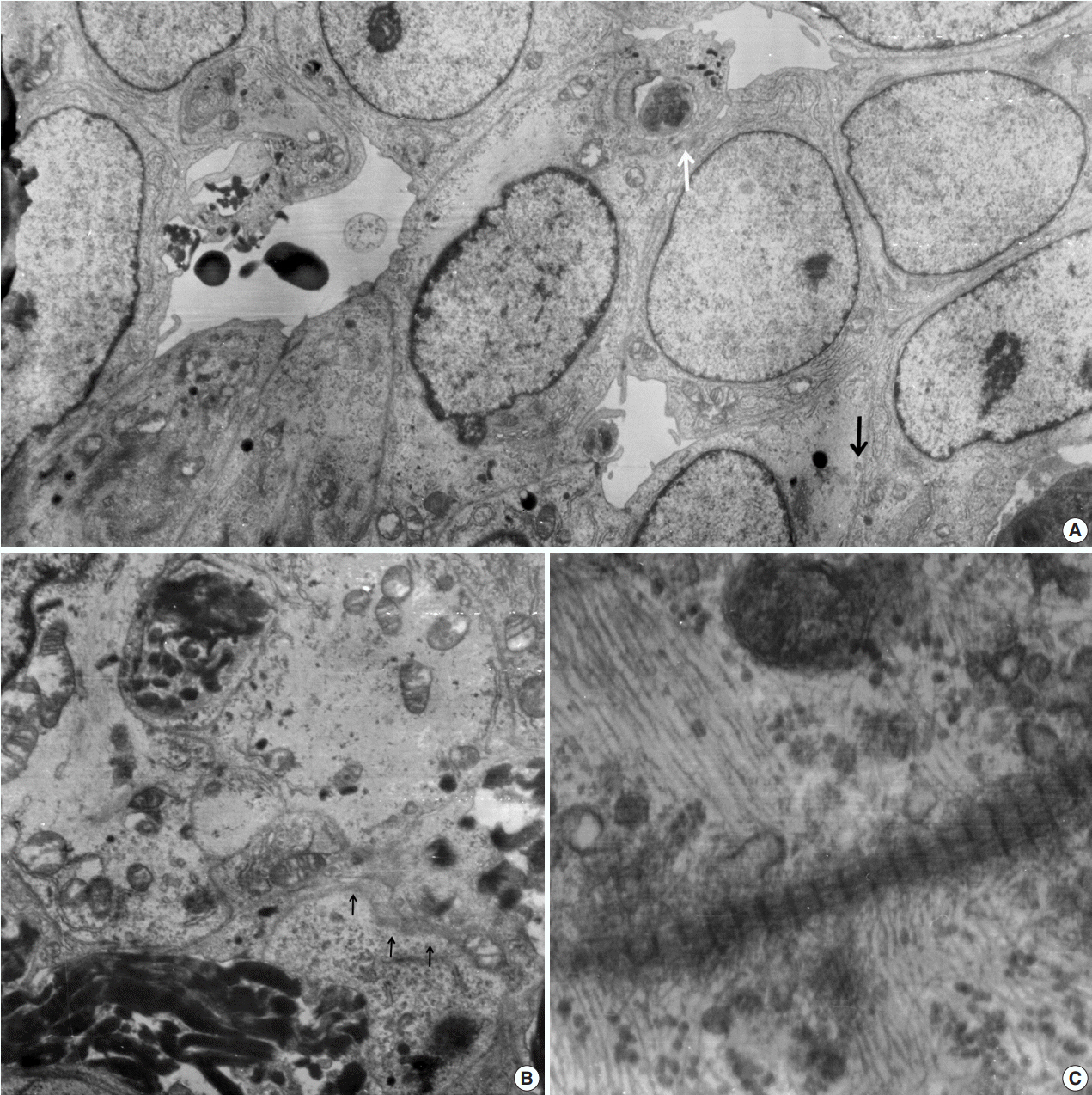
- A 51-year-old woman presented with severe dizziness. The brain magnetic resonance image revealed a 5.5 cm multiloculated mass with a thick rim in the left temporal lobe. Cytological examination of frozen diagnosis of the mass showed hypercellular sheets of round and rhabdoid cells in a hemorrhagic background, and two mitotic figures were observed. Histologically, the excised dura-based mass consisted of predominantly round cells with small foci of rhabdoid tumor cells in a pseudoalveolar pattern in a hemorrhagic background, and the cells showed nuclear positivity for signal transducer and activator of transcription 6 as well as frequent mitosis. The mass was diagnosed as a grade 3 solitary fibrous tumor (SFT)/hemangiopericytoma (HPC). The cytological diagnosis of SFT/HPC is challenging because of the heterogeneous cytological findings, such as histological heterogeneity, and because there are no standardized cytological criteria for malignant SFT/HPC. Cytological findings, such as singly scattered small cells, hypercellularity, rare ropy collagen, and round and rhabdoid cells with pseudoalveolar pattern, may assist in the diagnosis of malignant SFT/HPC.
MeSH Terms
Figure
Cited by 1 articles
-
Intraoperative frozen cytology of intraosseous cystic meningioma in the sphenoid bone
Na Rae Kim, Gie-Taek Yie
J Pathol Transl Med. 2020;54(6):508-512. doi: 10.4132/jptm.2020.05.21.
Reference
-
1. Bailey P, Cushing H, Eisenchardt L. Angioblastic meningioma. Arch Pathol Lab Med. 1928; 6:953–90.2. Tani E, Wejde J, Åström K, Wingmo IL, Larsson O, Haglund F. FNA cytology of solitary fibrous tumors and the diagnostic value of STAT6 immunocytochemistry. Cancer Cytopathol. 2018; 126:36–43.
Article3. Maekawa A, Kohashi K, Yamada Y, et al. A case of intracranial solitary fibrous tumor/hemangiopericytoma with dedifferentiated component. Neuropathology. 2015; 35:260–5.
Article4. Giannini G, Rushing EJ, Hainfellner JA, et al. Solitary fibrous tumour/haemangiopericytoma. In : Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4th ed. Solitary fibrous tumour/haemangiopericytoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO classification of tumours of the central nervous system. Lyon: IARC;2016. p. 249–54.5. Samal S, Kalra R, Sharma J, Singh I, Panda D, Ralli M. Comparison between crush/squash cytology and frozen section preparation in intraoperative diagnosis of central nervous system lesions. Oncol J India. 2017; 1:25–30.
Article6. Tihan T, Viglione M, Rosenblum MK, Olivi A, Burger PC. Solitary fibrous tumors in the central nervous system: a clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med. 2003; 127:432–9.7. Clayton AC, Salomao DR, Keeney GL, Nascimento AG. Solitary fibrous tumor: a study of cytologic features of six cases diagnosed by fine-needle aspiration. Diagn Cytopathol. 2001; 25:172–6.
Article8. Gill SS, Bharadwaj R. Cytomorphologic findings of hemangiopericytoma of the meninges: a case report. Indian J Pathol Microbiol. 2007; 50:422–5.9. Baliga M, Flowers R, Heard K, Siddiqi A, Akhtar I. Solitary fibrous tumor of the lung: a case report with a study of the aspiration biopsy, histopathology, immunohistochemistry, and autopsy findings. Diagn Cytopathol. 2007; 35:239–44.
Article10. Sandoh K, Ishida M, Okano K, et al. Cytological characteristics of meningeal solitary fibrous tumor metastatic to the lung: a case report with immunocytochemical analysis. Mol Clin Oncol. 2018; 9:17–20.11. Khanchel F, Driss M, Mrad K, Romdhane KB. Malignant solitary fibrous tumor in the extremity: cytopathologic findings. J Cytol. 2012; 29:139–41.
Article12. Bishop JA, Rekhtman N, Chun J, Wakely PE Jr, Ali SZ. Malignant solitary fibrous tumor: cytopathologic findings and differential diagnosis. Cancer Cytopathol. 2010; 118:83–9.13. Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF. Solitary fibrous tumor: a cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer. 1997; 81:116–21.14. Kwon JH, Song JS, Jung HW, Lee JS, Cho KJ. Malignant solitary fibrous tumor with heterologous rhabdomyosarcomatous differentiation: a case report. J Pathol Transl Med. 2017; 51:171–5.
Article15. Deb P, Kinra P, Bhatoe HS. Intraoperative cytology of central neurocytoma mimicking oligodendroglioma. J Cytol. 2011; 28:219–22.
Article16. Shetty KJ, Rao C, Prasad HL. Glomangiopericytoma versus solitary fibrous tumor: an omental tumor with unusual diagnostic dilemma. Indian J Surg Oncol. 2016; 7:475–8.
Article17. Naniwadekar MR, Jagtap SV, Kshirsagar AY, Shinagare SA, Tata HR, Sahoo K. Fine needle aspiration diagnosis of carotid body tumor in a case of multiple paragangliomas presenting with facial palsy: a case report. Acta Cytol. 2010; 54:635–9.18. Xiao GQ, Burstein DE. Cytologic findings of rhabdoid meningioma in cerebrospinal fluid. Acta Cytol. 2008; 52:118–9.
Article19. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–20.
Article20. Stacchiotti S, Tortoreto M, Bozzi F, et al. Dacarbazine in solitary fibrous tumor: a case series analysis and preclinical evidence vis-avis temozolomide and antiangiogenics. Clin Cancer Res. 2013; 19:5192–201.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Meningeal Solitary Fibrous Tumor
- Meningeal Hemangiopericytoma in a Newborn: A Case Report
- Cystic Solitary Fibrous Tumor Arising From the Left Occipital Meninges: A Case Report
- Dynamic Contrast-Enhanced CT Findings of a Extrapleural Solitary Fibrous Tumor in the Spleen: A Case Report and Literature Review
- Is a Solitary Fibrous Tumor in the External Auditory Canal Benign?