Anesth Pain Med.
2019 Apr;14(2):187-192. 10.17085/apm.2019.14.2.187.
Relationship between PaOâ‚‚/FiOâ‚‚ and number of regions with B-line on transthoracic lung ultrasound: a prospective, observational study
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 2Department of Policy Research Affairs, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 3Department of Anesthesiology and Pain Medicine and Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. KIMJY@yuhs.ac
- 4Department of Anesthesia and Pain Medicine, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2447967
- DOI: http://doi.org/10.17085/apm.2019.14.2.187
Abstract
- BACKGROUND
Aeration of the lungs must be monitored during general anesthesia because of the possibility of postsurgical pulmonary complications. The aim of this study was to compare PaOâ‚‚/FiOâ‚‚ and the number of regions with B-line on transthoracic lung ultrasonography (TLU) between the postinduction and postsurgical periods.
METHODS
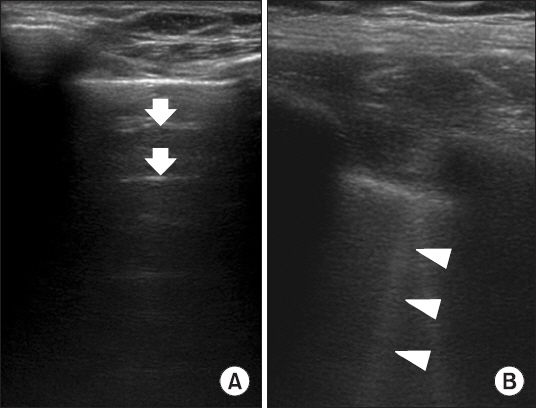
Twenty-six adult patients undergoing major abdominal surgery were enrolled. Arterial blood gas analysis and TLU were performed 30 min after the induction of anesthesia (postinduction) and after skin closure (postsurgical period) while patients were under mechanical ventilation. TLU was performed in 12 regions (anterior, lateral, and posterior in the upper and lower regions of both lungs). The number of regions with B-line was counted.
RESULTS
Compared with postinduction values, the number of regions with B-line on TLU was increased in the postsurgical period (0.3 ± 0.5 to 1.3 ± 1.2, P < 0.001); however, PaOâ‚‚/FiOâ‚‚ did not significantly differ (421.3 ± 95.8 to 425.2 ± 86.0, P = 0.765). The change in PaOâ‚‚/FiOâ‚‚ (postinduction-postsurgical period) was significantly higher in Group B than in Group A (P = 0.028).
CONCLUSIONS
Although the number of regions with B-line on TLU was increased in the postsurgical period, lung oxygenation did not differ, based on the main assessment in this study. In contrast, patients with an increased number of regions with B-line tended to show a reduction in PaOâ‚‚/FiOâ‚‚ during the postsurgical period. Further study seems necessary to establish the number of regions with B-line on TLU as a tool for evaluation of perioperative oxygenation.
Keyword
MeSH Terms
Figure
Reference
-
1. Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Heden-stierna G. Prevention of atelectasis during general anaesthesia. Lancet. 1995; 345:1387–91. DOI: 10.1016/S0140-6736(95)92595-3.2. Magnusson L, Spahn DR. New concepts of atelectasis during general anaesthesia. Br J Anaesth. 2003; 91:61–72. DOI: 10.1093/bja/aeg085. PMID: 12821566.3. Restrepo RD, Braverman J. Current challenges in the recognition, prevention and treatment of perioperative pulmonary atelectasis. Expert Rev Respir Med. 2015; 9:97–107. DOI: 10.1586/17476348.2015.996134. PMID: 25541220.4. Akça O, Podolsky A, Eisenhuber E, Panzer O, Hetz H, Lampl K, et al. Comparable postoperative pulmonary atelectasis in patients given 30% or 80% oxygen during and 2 hours after colon resection. Anesthesiology. 1999; 91:991–8. DOI: 10.1097/00000542-199910000-00019.5. Benoît Z, Wicky S, Fischer JF, Frascarolo P, Chapuis C, Spahn DR, et al. The effect of increased FIO(2) before tracheal extubation on postoperative atelectasis. Anesth Analg. 2002; 95:1777–81. DOI: 10.1097/00000539-200212000-00058.6. Rothen HU, Sporre B, Engberg G, Wegenius G, Högman M, Hedenstierna G. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology. 1995; 82:832–42. DOI: 10.1097/00000542-199504000-00004.7. Staehr AK, Meyhoff CS, Henneberg SW, Christensen PL, Rasmussen LS. Influence of perioperative oxygen fraction on pulmonary function after abdominal surgery: a randomized controlled trial. BMC Res Notes. 2012; 5:383. DOI: 10.1186/1756-0500-5-383. PMID: 22840231. PMCID: PMC3434073.8. Acosta CM, Maidana GA, Jacovitti D, Belaunzarán A, Cereceda S, Rae E, et al. Accuracy of transthoracic lung ultrasound for diagnosing anesthesia-induced atelectasis in children. Anesthesiology. 2014; 120:1370–9. DOI: 10.1097/ALN.0000000000000231. PMID: 24662376.9. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014; 12:25. DOI: 10.1186/1476-7120-12-25. PMID: 24993976. PMCID: PMC4098927.10. Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 2009; 135:1421–5. DOI: 10.1378/chest.08-2281. PMID: 19225063.11. Monastesse A, Girard F, Massicotte N, Chartrand-Lefebvre C, Girard M. Lung ultrasonography for the assessment of perioperative atelectasis: a pilot feasibility study. Anesth Analg. 2017; 124:494–504. DOI: 10.1213/ANE.0000000000001603. PMID: 27669555.12. Dietrich CF, Mathis G, Blaivas M, Volpicelli G, Seibel A, Wastl D, et al. Lung B-line artefacts and their use. J Thorac Dis. 2016; 8:1356–65. DOI: 10.21037/jtd.2016.04.55. PMID: 27293860. PMCID: PMC4885976.13. Casati A, Comotti L, Tommasino C, Leggieri C, Bignami E, Tarantino F, et al. Effects of pneumoperitoneum and reverse Trendelenburg position on cardiopulmonary function in morbidly obese patients receiving laparoscopic gastric banding. Eur J Anaesthesiol. 2000; 17:300–5. DOI: 10.1097/00003643-200005000-00005.14. Yu X, Zhai Z, Zhao Y, Zhu Z, Tong J, Yan J, et al. Performance of lung ultrasound in detecting peri-operative atelectasis after general anesthesia. Ultrasound Med Biol. 2016; 42:2775–84. DOI: 10.1016/j.ultrasmedbio.2016.06.010. PMID: 27639431.15. Soldati G, Inchingolo R, Smargiassi A, Sher S, Nenna R, Inchingolo CD, et al. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol. 2012; 38:1169–79. DOI: 10.1016/j.ultrasmedbio.2012.03.001. PMID: 22579543.16. Futier E, Constantin JM, Petit A, Jung B, Kwiatkowski F, Duclos M, et al. Positive end-expiratory pressure improves end-expiratory lung volume but not oxygenation after induction of anaesthesia. Eur J Anaesthesiol. 2010; 27:508–13. DOI: 10.1097/EJA.0b013e3283398806. PMID: 20404729.17. Agarwal A, Singh PK, Dhiraj S, Pandey CM, Singh U. Oxygen in air (FiO2 0.4) improves gas exchange in young healthy patients during general anesthesia. Can J Anaesth. 2002; 49:1040–3. DOI: 10.1007/BF03017898. PMID: 12477674.18. Hedenstierna G. Oxygen and anesthesia: what lung do we deliver to the post-operative ward? Acta Anaesthesiol Scand. 2012; 56:675–85. DOI: 10.1111/j.1399-6576.2012.02689.x. PMID: 22471648.19. Hedenstierna G, Strandberg A, Brismar B, Lundquist H, Svens- son L, Tokics L. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology. 1985; 62:247–54. DOI: 10.1097/00000542-198503000-00007.20. Mancini M, Zavala E, Mancebo J, Fernandez C, Barberà JA, Rossi A, et al. Mechanisms of pulmonary gas exchange improvement during a protective ventilatory strategy in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001; 164:1448–53. DOI: 10.1164/ajrccm.164.8.9911034. PMID: 11704594.21. Hegeman MA, Hennus MP, Heijnen CJ, Specht PA, Lachmann B, Jansen NJ, et al. Ventilator-induced endothelial activation and inflammation in the lung and distal organs. Crit Care. 2009; 13:R182. DOI: 10.1186/cc8168. PMID: 19917112. PMCID: PMC2811914.22. Murray JF. Pulmonary edema: pathophysiology and diagnosis. Int J Tuberc Lung Dis. 2011; 15:155–60. DOI: 10.5588/ijtld.11.0324-2.23. Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005; 102:838–54. DOI: 10.1097/00000542-200504000-00021. PMID: 15791115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transthoracic Needle Biopsy: How to Maximize Diagnostic Accuracy and Minimize Complications
- Ultrasonography-guided Transthoracic Cutting Biopsy of Pulmonary Lesion: Diagnostic Benefits and Safety
- Pediatric lung ultrasound: its role in the perioperative period
- Pulmonary Artery Stenosis due to Lung Carcinoma: A Rare Cause of Dyspnea
- The Effect of Orthopaedic Operations upon Postoperative Arterial Oxygen Tension