Korean J Physiol Pharmacol.
2019 Jan;23(1):63-70. 10.4196/kjpp.2019.23.1.63.
Computational analysis of the electromechanical performance of mitral valve cerclage annuloplasty using a patient-specific ventricular model
- Affiliations
-
- 1Department of Mechanical and Biomedical Engineering, Kangwon National University, Chuncheon 24340, Korea. ebshim@kangwon.ac.kr
- 2Department of Cardiology, College of Medicine, Pusan National University, Busan 46241, Korea. junehongk@gmail.com
- KMID: 2443617
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.63
Abstract
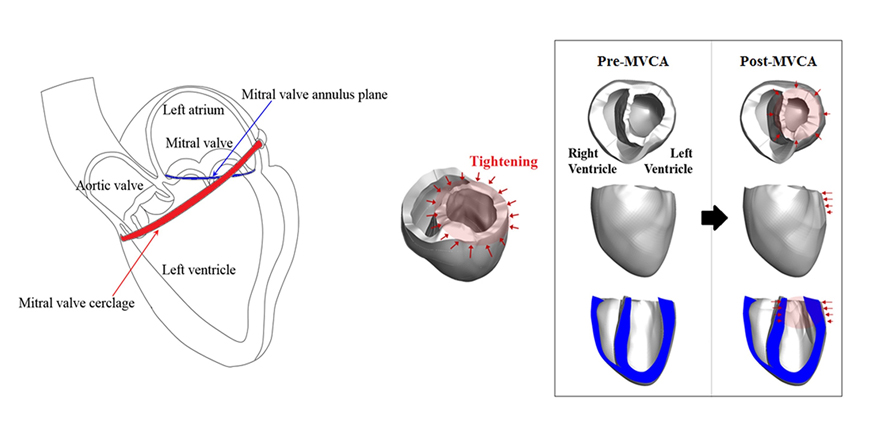
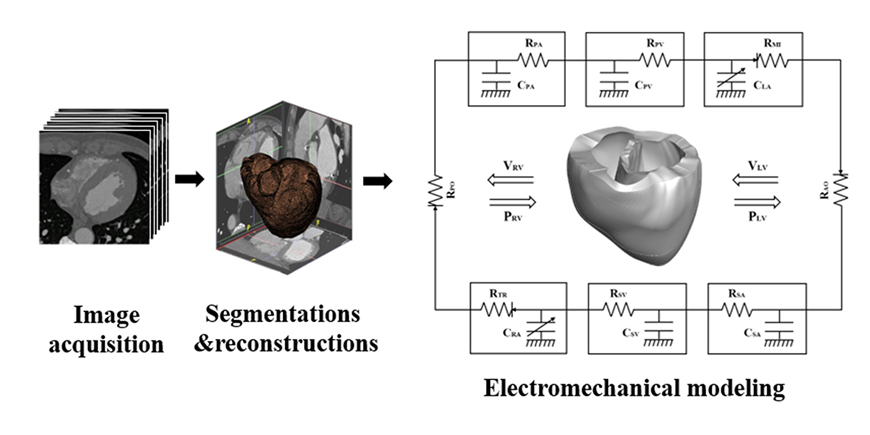
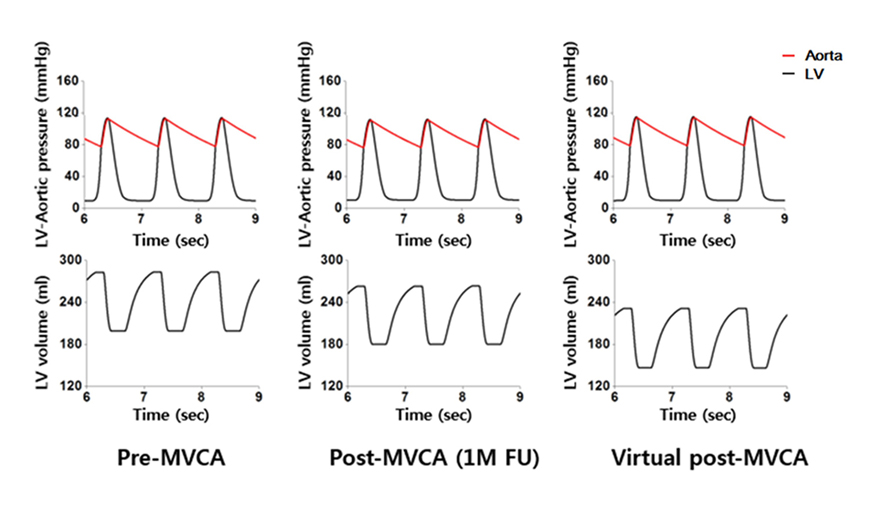
- We aimed to propose a novel computational approach to predict the electromechanical performance of pre- and post-mitral valve cerclage annuloplasty (MVCA). Furthermore, we tested a virtual estimation method to optimize the left ventricular basement tightening scheme using a pre-MVCA computer model. The present model combines the three-dimensional (3D) electromechanics of the ventricles with the vascular hemodynamics implemented in a lumped parameter model. 3D models of pre- and post-MVCA were reconstructed from the computed tomography (CT) images of two patients and simulated by solving the electromechanical-governing equations with the finite element method. Computed results indicate that reduction of the dilated heart chambers volume (reverse remodeling) appears to be dependent on ventricular stress distribution. Reduced ventricular stresses in the basement after MVCA treatment were observed in the patients who showed reverse remodeling of heart during follow up over 6 months. In the case who failed to show reverse remodeling after MVCA, more virtual tightening of the ventricular basement diameter than the actual model can induce stress unloading, aiding in heart recovery. The simulation result that virtual tightening of the ventricular basement resulted in a marked increase of myocardial stress unloading provides in silico evidence for a functional impact of MVCA treatment on cardiac mechanics and post-operative heart recovery. This technique contributes to establishing a pre-operative virtual rehearsal procedure before MVCA treatment by using patient-specific cardiac electromechanical modeling of pre-MVCA.
Keyword
Figure
Cited by 1 articles
-
Digital heart for life
Yin Hua Zhang
Korean J Physiol Pharmacol. 2019;23(5):291-293. doi: 10.4196/kjpp.2019.23.5.291.
Reference
-
1. Herrmann HC, Maisano F. Transcatheter therapy of mitral regurgitation. Circulation. 2014; 130:1712–1722.
Article2. Kim JH, Kocaturk O, Ozturk C, Faranesh AZ, Sonmez M, Sampath S, Saikus CE, Kim AH, Raman VK, Derbyshire JA, Schenke WH, Wright VJ, Berry C, McVeigh ER, Lederman RJ. Mitral cerclage annuloplasty, a novel transcatheter treatment for secondary mitral valve regurgitation: initial results in swine. J Am Coll Cardiol. 2009; 54:638–651.3. Kim JH, Sung SC, Chon MK, Kim JO, Lee SH, Lee SY, Je HG, Choo KS, Hwang JM, Kim JS, Park YH, Chun KJ, Kim CM. Mitral loop cerclage as a variant form of mitral cerclage annuloplasty that adds a device (CSTV) for preventing potential complications: a preclinical proof of concept and feasibility study. EuroIntervention. 2016; 11:e1669–e1679.
Article4. Park YH, Chon MK, Lederman RJ, Sung SC, Je HG, Choo KS, Lee SH, Shin ES, Kim JS, Hwang KW, Lee SY, Chun KJ, Kim CM, Kim JH. Mitral loop cerclage annuloplasty for secondary mitral regurgitation: first human results. JACC Cardiovasc Interv. 2017; 10:597–610.5. Lim KM, Constantino J, Gurev V, Zhu R, Shim EB, Trayanova NA. Comparison of the effects of continuous and pulsatile left ventricular-assist devices on ventricular unloading using a cardiac electromechanics model. J Physiol Sci. 2012; 62:11–19.
Article6. Lim KM, Hong SB, Lee BK, Shim EB, Trayanova N. Computational analysis of the effect of valvular regurgitation on ventricular mechanics using a 3D electromechanics model. J Physiol Sci. 2015; 65:159–164.
Article7. Yuniarti AR, Lim KM. The effect of electrical conductivity of myocardium on cardiac pumping efficacy: a computational study. Biomed Eng Online. 2017; 16:11.
Article8. Trayanova NA, Constantino J, Gurev V. Electromechanical models of the ventricles. Am J Physiol Heart Circ Physiol. 2011; 301:H279–H286.
Article9. Watanabe H, Hisada T, Sugiura S, Okada J, Fukunari H. Computer simulation of blood flow, left ventricular wall motion and their interrelationship by fluid-structure interaction finite element method. JSME. 2002; 45:1003–1012.
Article10. Kerckhoffs RCP, Healy SN, Usyk TP, McCulloch AD. Computational methods for cardiac electromechanics. Proc IEEE. 2006; 94:769–783.
Article11. Gurev V, Lee T, Constantino J, Arevalo H, Trayanova NA. Models of cardiac electromechanics based on individual hearts imaging data: image-based electromechanical models of the heart. Biomech Model Mechanobiol. 2011; 10:295–306.12. ten Tusscher KH, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol. 2006; 291:H1088–H1100.
Article13. Rice JJ, Wang F, Bers DM, de Tombe PP. Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary differential equations. Biophys J. 2008; 95:2368–2390.
Article14. Kono T, Sabbah HN, Stein PD, Brymer JF, Khaja F. Left ventricular shape as a determinant of functional mitral regurgitation in patients with severe heart failure secondary to either coronary artery disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1991; 68:355–359.
Article15. Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000; 102:470–479.16. Sabbah HN, Kono T, Rosman H, Jafri S, Stein PD, Goldstein S. Left ventricular shape: a factor in the etiology of functional mitral regurgitation in heart failure. Am Heart J. 1992; 123:961–966.
Article17. Tibayan FA, Rodriguez F, Langer F, Liang D, Daughters GT, Ingels NB Jr, Miller DC. Undersized mitral annuloplasty alters left ventricular shape during acute ischemic mitral regurgitation. Circulation. 2004; 110:11 Suppl 1. II98–II102.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Annular Plication Technique to Facilitate Sliding Annuloplasty in a Marfan's Syndrome Patient
- Perforated Mitral Valve Aneurysm in the Posterior Leaflet without Infective Endocarditis
- Assessment of Mitral Valve Complex by Three-Dimensional Echocardiography: Therapeutic Strategy for Functional Mitral Regurgitation
- A Clinical Study of Mitral Valve Repair for the Treatment of Mitral Valve Insufficiency
- Congenital Double-Orifice Mitral Valve with Mitral Valve Prolapse and Severe Mitral Regurgitation