Korean J Physiol Pharmacol.
2019 Jan;23(1):47-54. 10.4196/kjpp.2019.23.1.47.
SPA0355 prevents ovariectomy-induced bone loss in mice
- Affiliations
-
- 1Department of Surgery, Chonbuk National University Medical School, Jeonju 54896, Korea.
- 2Department of Orthopaedic Surgery, Chonbuk National University Medical School, Jeonju 54896, Korea.
- 3Department of Biochemistry, Chonbuk National University Medical School, Jeonju 54896, Korea. bhpark@jbnu.ac.kr
- 4Department of Cognitive Science, Case Western Reserve University, Cleveland, OH 44106, USA.
- 5Department of Laboratory Medicine, Chonbuk National University Medical School, Jeonju 54896, Korea.
- 6College of Pharmacy, Sookmyung Women's University, Seoul 04310, Korea. rjeon@sm.ac.kr
- KMID: 2443615
- DOI: http://doi.org/10.4196/kjpp.2019.23.1.47
Abstract
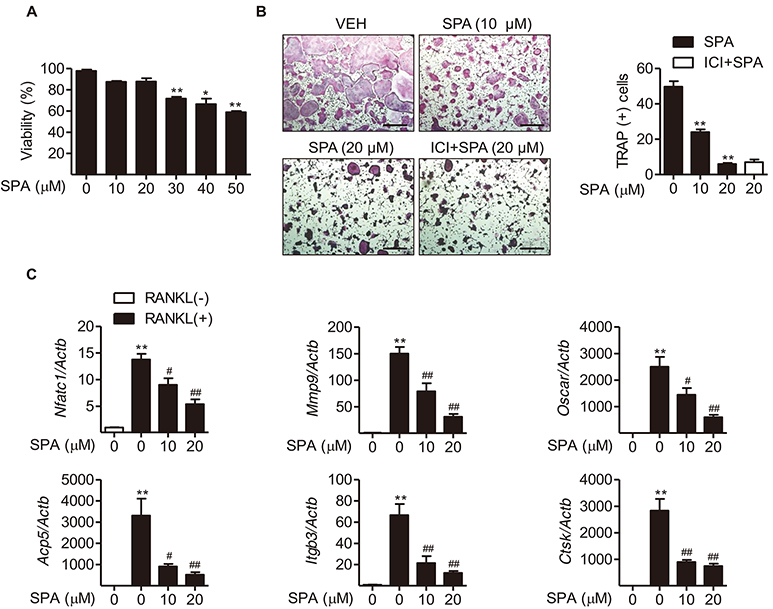
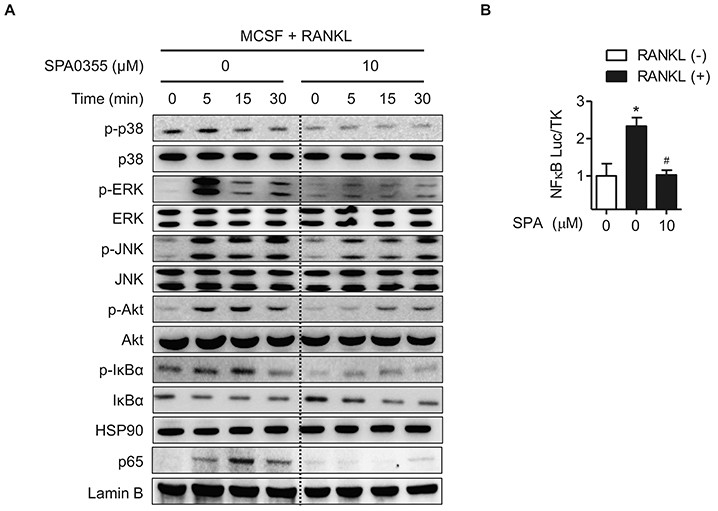
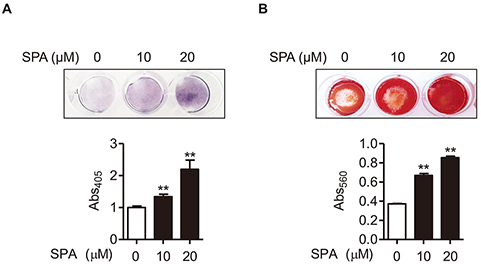
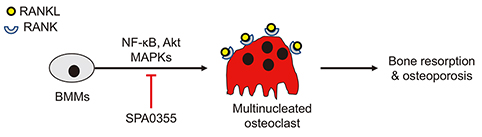
- Estrogen withdrawal in post-menopausal women leads to overactivation of osteoclasts, which contributes to the development of osteoporosis. Inflammatory cytokines are known as one of mechanisms of osteoclast activation after estrogen deficiency. SPA0355 is a thiourea derivative that has been investigated for its antioxidant and anti-inflammatory activities. However, its efficacy in bone resorption has not been previously investigated. The aim of this study was to investigate the impact of SPA0355 on the development of osteoporosis and to explore its mode of action. In vitro experiments showed that SPA0355 inhibited receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis in primary bone marrow-derived macrophages. This effect appears to be independent of estrogen receptor activation as ICI 180,782 failed to abrogate its effects on osteoclasts. Further signaling studies revealed that SPA0355 suppressed activation of the MAPKs, Akt, and NF-κB pathways. SPA0355 also increased osteoblastic differentiation, as evidenced by its effects on alkaline phosphatase activity and mineralization nodule formation. Intraperitoneal administration of SPA0355 to ovariectomized mice prevented bone loss, as verified by three-dimensional images and bone morphometric parameters derived from µCT analysis. Noticeably, SPA0355 did not show hepatotoxicity and nephrotoxicity and also had little effect on hematological parameters. Taken together, the results indicate that SPA0355 may protect against bone loss in ovariectomized mice by stimulation of osteoblast differentiation and by inhibition of osteoclast resorption. Therefore, SPA0355 is a safe and potential candidate for management of postmenopausal osteoporosis.
MeSH Terms
Figure
Reference
-
1. Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010; 21:369–374.
Article2. Fujiwara Y, Piemontese M, Liu Y, Thostenson JD, Xiong J, O'Brien CA. RANKL (Receptor Activator of NFκB Ligand) produced by osteocytes is required for the increase in B cells and bone loss caused by estrogen deficiency in mice. J Biol Chem. 2016; 291:24838–24850.
Article3. Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J Biol Chem. 1999; 274:7724–7731.4. Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003; 111:1221–1230.
Article5. Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J Biol Chem. 2000; 275:31155–31161.6. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.
Article7. Lee YR, Hwang JK, Lee HS, Cheon YJ, Ryu JH, Lee SI, Kwak HB, Lee SM, Kim JS, Park JW, Jeon R, Park BH. SPA0355, a thiourea analogue, inhibits inflammatory responses and joint destruction in fibroblast-like synoviocytes and mice with collagen-induced arthritis. Br J Pharmacol. 2011; 164:794–806.
Article8. Bae UJ, Song MY, Jang HY, Gim HJ, Ryu JH, Lee SM, Jeon R, Park BH. The efficacy of SPA0355 in protecting β cells in isolated pancreatic islets and in a murine experimental model of type 1 diabetes. Exp Mol Med. 2013; 45:e51.
Article9. Bae UJ, Yang JD, Ka SO, Koo JH, Woo SJ, Lee YR, Yu HC, Cho BH, Zhao HY, Ryu JH, Lee SM, Jeon R, Park BH. SPA0355 attenuates ischemia/reperfusion-induced liver injury in mice. Exp Mol Med. 2014; 46:e109.
Article10. Jang HY, Jeon R, Kang KW, Song MY, Lim JM, Lee E, Ryu JH, Lee SM, Park BH. SPA0355 suppresses T-cell responses and reduces airway inflammation in mice. Eur J Pharmacol. 2014; 745:19–28.
Article11. Lee Y, Ka SO, Cha HN, Chae YN, Kim MK, Park SY, Bae EJ, Park BH. Myeloid sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype. Diabetes. 2017; 66:2659–2668.
Article12. Cao JJ, Gregoire BR, Sun L, Song S. Alpha-1 antitrypsin reduces ovariectomy-induced bone loss in mice. Ann N Y Acad Sci. 2011; 1240:E31–E35.
Article13. Abuohashish HM, Ahmed MM, Al-Rejaie SS, Eltahir KE. The antidepressant bupropion exerts alleviating properties in an ovariectomized osteoporotic rat model. Acta Pharmacol Sin. 2015; 36:209–220.
Article14. Santen RJ, Kagan R, Altomare CJ, Komm B, Mirkin S, Taylor HS. Current and evolving approaches to individualizing estrogen receptor-based therapy for menopausal women. J Clin Endocrinol Metab. 2014; 99:733–747.
Article15. Zhao R. Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci. 2012; 9:825–832.
Article16. Rufus P, Mohamed N, Shuid AN. Beneficial effects of traditional Chinese medicine on the treatment of osteoporosis on ovariectomised rat models. Curr Drug Targets. 2013; 14:1689–1693.
Article17. Wijkmans J, Gossen J. Inhibitors of cathepsin K: a patent review (2004 – 2010). Expert Opin Ther Pat. 2011; 21:1611–1629.
Article18. Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010; 31:266–300.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 1,3-Dibenzyl-5-Fluorouracil Prevents Ovariectomy-Induced Bone Loss by Suppressing Osteoclast Differentiation
- Inhibitory Effect of SPA0355, a Thiourea Analogue, on Inflammation and Alveolar Bone Loss in Rats with Ligature-Induced Periodontitis
- A Vitronectin-Derived Peptide Restores Ovariectomy-Induced Bone Loss by Dual Regulation of Bone Remodeling
- The Effect of Intermittent Artificial Gravity (IAG) on Osteoporosis Induced by Ovariectomy in Rats - Histomorphometric Analysis by Using 3D Micro-CT -
- Platinum nanoparticles reduce ovariectomy-induced bone loss by decreasing osteoclastogenesis