Dement Neurocogn Disord.
2016 Dec;15(4):147-152. 10.12779/dnd.2016.15.4.147.
Longitudinal Cerebral Perfusion Changes in Parkinson's Disease with Subjective Cognitive Impairment
- Affiliations
-
- 1Department of Radiology, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon, Korea.
- 2Department of Nursing, U1 University, Yeongdong, Korea.
- 3Department of Neurology, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon, Korea. siuy@catholic.ac.kr
- 4Department of Brain and Cognitive Sciences, Ewha Womans University, Seoul, Korea.
- 5Department of Neurology, Veterans Hospital, Seoul Medical Center, Seoul, Korea.
- KMID: 2442841
- DOI: http://doi.org/10.12779/dnd.2016.15.4.147
Abstract
- BACKGROUND AND PURPOSE
Although subjective cognitive impairment (SCI) is often accompanied by Parkinson's disease (PD) and may predict the development of mild cognitive impairment or dementia, longitudinal brain perfusion changes in PD patients with SCI remain to be elucidated. The current prospective study examined cerebral perfusion changes in PD patients with SCI using technetium-99m hexamethylpropylene amine oxime single photon emission computed tomography (SPECT).
METHODS
Among 53 PD patients at baseline, 30 patients were classified into the PD with SCI group and 23 patients were assigned to the PD without SCI group. The mean follow-up interval was 2.3±0.9 years. The Mini-Mental State Examination, Clinical Dementia Rating, and Global Deterioration Scale were used to assess impairments in cognitive function. Brain SPECT images were acquired at baseline and follow-up.
RESULTS
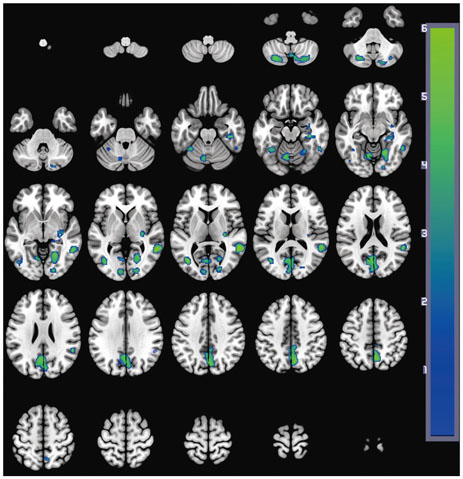
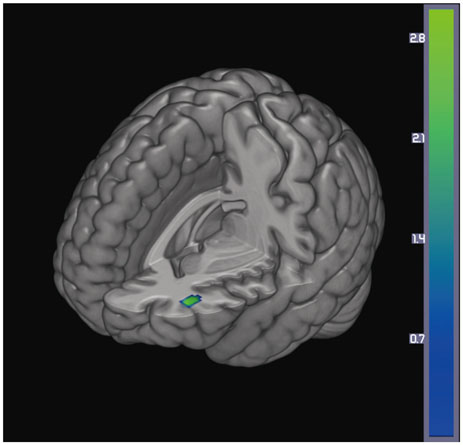
Significant differences between the two groups were not found for demographic variables, PD severity, or cognitive function at either baseline or follow-up. At baseline, the PD with SCI group showed decreased perfusion in the left angular gyrus compared to the PD without SCI group. Longitudinal analysis revealed widespread perfusion reductions primarily in the bilateral temporo-parieto-occipital areas and cerebellum in the PD with SCI group. Relative to the PD without SCI group, an excessive decrease of perfusion was found in the left middle frontal gyrus of the PD with SCI patients.
CONCLUSIONS
Our findings suggest that perfusion deficits in the middle frontal area may play an important role in the pathophysiology of SCI in PD.
Keyword
MeSH Terms
Figure
Reference
-
1. Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010; 9:1200–1213.
Article2. Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003; 60:387–392.
Article3. Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology. 2001; 56:730–736.
Article4. Stewart R. Subjective cognitive impairment. Curr Opin Psychiatry. 2012; 25:445–450.
Article5. Erro R, Santangelo G, Barone P, Picillo M, Amboni M, Longo K, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol. 2014; 27:276–281.
Article6. Hong JY, Sunwoo MK, Chung SJ, Ham JH, Lee JE, Sohn YH, et al. Subjective cognitive decline predicts future deterioration in cognitively normal patients with Parkinson's disease. Neurobiol Aging. 2014; 35:1739–1743.
Article7. Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement. 2008; 4:1 Suppl 1. S98–S108.
Article8. Hong JY, Lee JE, Sohn YH, Lee PH. Neurocognitive and atrophic patterns in Parkinson's disease based on subjective memory complaints. J Neurol. 2012; 259:1706–1712.
Article9. Hong JY, Yun HJ, Sunwoo MK, Ham JH, Lee JM, Sohn YH, et al. Cognitive and cortical thinning patterns of subjective cognitive decline in patients with and without Parkinson's disease. Parkinsonism Relat Disord. 2014; 20:999–1003.
Article10. Song IU, Kim JS, Chung SW, Lee KS, Oh JK, Chung YA. Early detection of subjective memory impairment in Parkinson's disease using cerebral perfusion SPECT. Biomed Mater Eng. 2014; 24:3405–3410.
Article11. Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson's disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007; 78:254–259.
Article12. Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH. The pattern of cortical atrophy in patients with Parkinson's disease according to cognitive status. Mov Disord. 2011; 26:289–296.
Article13. Amboni M, Tessitore A, Esposito F, Santangelo G, Picillo M, Vitale C, et al. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson's disease. J Neurol. 2015; 262:425–434.
Article14. Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008; 70(16 Pt 2):1470–1477.
Article15. Caviness JN, Driver-Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007; 22:1272–1277.
Article16. Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G;. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009; 72:1121–1126.
Article17. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967; 17:427–442.
Article18. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989; 39:1159–1165.19. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The validity of the Korean Version of Global Deterioration Scale. J Korean Neurol Assoc. 2002; 20:612–617.20. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.21. Yakushev I, Hammers A, Fellgiebel A, Schmidtmann I, Scheurich A, Buchholz HG, et al. SPM-based count normalization provides excellent discrimination of mild Alzheimer's disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009; 44:43–50.
Article22. Borghammer P, Aanerud J, Gjedde A. Data-driven intensity normalization of PET group comparison studies is superior to global mean normalization. Neuroimage. 2009; 46:981–988.
Article23. Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008; 63:609–618.
Article24. Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006; 16:1418–1430.
Article25. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013; 19:43–61.26. Borghammer P, Chakravarty M, Jonsdottir KY, Sato N, Matsuda H, Ito K, et al. Cortical hypometabolism and hypoperfusion in Parkinson's disease is extensive: probably even at early disease stages. Brain Struct Funct. 2010; 214:303–317.
Article27. Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O'Brien JT. Regional cerebral blood flow in Parkinson's disease with and without dementia. Neuroimage. 2003; 20:1309–1319.
Article28. Melzer TR, Watts R, MacAskill MR, Pearson JF, Rüeger S, Pitcher TL, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson's disease. Brain. 2011; 134(Pt 3):845–855.
Article29. Leenders KL, Wolfson L, Gibbs JM, Wise RJ, Causon R, Jones T, et al. The effects of L-DOPA on regional cerebral blood flow and oxygen metabolism in patients with Parkinson's disease. Brain. 1985; 108(Pt 1):171–191.
Article30. Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson's disease. J Nucl Med. 1996; 37:1115–1122.31. Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. 2013; 136(Pt 3):696–709.
Article32. Hosokai Y, Nishio Y, Hirayama K, Takeda A, Ishioka T, Sawada Y, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson's disease with and without mild cognitive impairment. Mov Disord. 2009; 24:854–862.
Article33. Jahn H. Memory loss in Alzheimer's disease. Dialogues Clin Neurosci. 2013; 15:445–454.
Article34. Jeong HS, Park JS, Song IU, Chung YA, Rhie SJ. Changes in cognitive function and brain glucose metabolism in elderly women with subjective memory impairment: a 24-month prospective pilot study. Acta Neurol Scand. 2017; 135:108–114.
Article35. Brück A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004; 75:1467–1469.
Article36. Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006; 31:496–504.
Article37. Hwang KJ, Kim EH, Kim YJ, Hong SB. Frequency of depression and suicidality in patients with neurological disorders: epilepsy, Parkinson's disease, and ischemic stroke. J Korean Neurol Assoc. 2016; 34:193–200.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Subjective Cognitive Complaints in Cognitively Normal Patients With Parkinson’s Disease: A Systematic Review
- Reliability and Validity of the Subjective Cognitive Complaints Questionnaire for Parkinson’s Disease (SCCQ-PD)
- Subjective Cognitive Complaints and Objective Cognitive Impairment in Parkinson's Disease
- Constipation is Associated With Mild Cognitive Impairment in Patients With de novo Parkinson’s Disease
- Fasting Plasma Glucose Levels and Longitudinal Motor and Cognitive Outcomes in Parkinson’s Disease Patients