Dement Neurocogn Disord.
2017 Dec;16(4):121-127. 10.12779/dnd.2017.16.4.121.
Neural Stem Cell Death Mechanisms Induced by Amyloid Beta
- Affiliations
-
- 1Department of Neurology, Hanyang University Guri Hospital, Guri, Korea. chj@hanyang.ac.kr
- KMID: 2442729
- DOI: http://doi.org/10.12779/dnd.2017.16.4.121
Abstract
- BACKGROUND AND PURPOSE
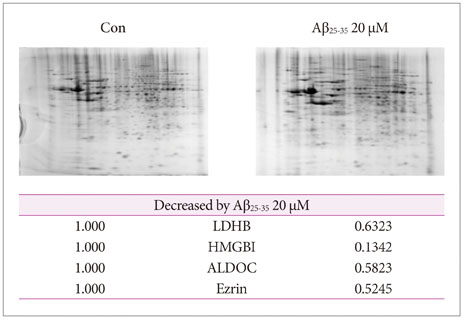
Amyloid beta (Aβ) is the main component of amyloid plaques, which are deposited in the brains of patients with Alzheimer's disease (AD). Biochemical and animal studies support the central role of Aβ in AD pathogenesis. Despite several investigations focused on the pathogenic mechanisms of Aβ, it is still unclear how Aβ accumulates in the central nervous system and subsequently initiates the disease at the cellular level. In this study, we investigated the pathogenic mechanisms of Aβ using proteomics and antibody microarrays.
METHODS
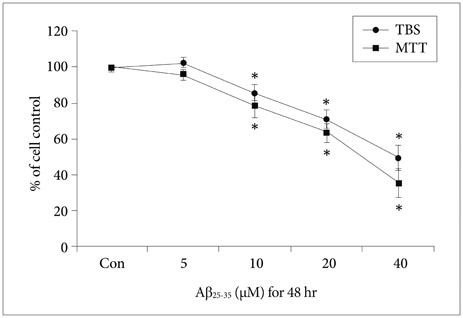
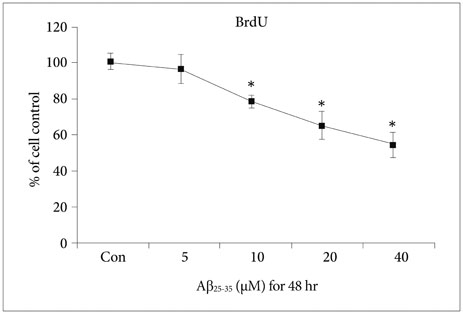
To evaluate the effect of Aβ on neural stem cells (NSCs), we treated primary cultured cortical NSCs with several doses of Aβ for 48 h. A 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay, trypan blue staining, and bromodeoxyuridine cell proliferation assay were performed. We detected several intracellular proteins that may be associated with Aβ by proteomics and Western blotting analysis.
RESULTS
Various viability tests showed that Aβ decreased NSCs viability and cell proliferation in a concentration-dependent manner. Aβ treatment significantly decreased lactate dehydrogenase B, high-mobility group box 1, aldolase C, Ezrin, and survival signals including phosphorylated phosphoinositide 3-kinase, Akt, and glycogen synthase kinase-3β.
CONCLUSIONS
These results suggest that several factors determined by proteomics and Western blot hold the clue to Aβ pathogenesis. Further studies are required to investigate the role of these factors.
Keyword
MeSH Terms
-
Alzheimer Disease
Amyloid*
Animals
Blotting, Western
Brain
Bromodeoxyuridine
Cell Proliferation
Central Nervous System
Fructose-Bisphosphate Aldolase
Glycogen Synthase
Humans
L-Lactate Dehydrogenase
Neural Stem Cells*
Plaque, Amyloid
Proteomics
Trypan Blue
Amyloid
Bromodeoxyuridine
Fructose-Bisphosphate Aldolase
Glycogen Synthase
L-Lactate Dehydrogenase
Trypan Blue
Figure
Reference
-
1. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005; 366:2112–2117.
Article2. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011; 7:137–152.
Article3. Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006; 67:1340–1352.
Article4. Lewczuk P, Mroczko B, Fagan A, Kornhuber J. Biomarkers of Alzheimer's disease and mild cognitive impairment: a current perspective. Adv Med Sci. 2015; 60:76–82.
Article5. Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol. 2015; 129:1–19.
Article6. Vetrivel KS, Thinakaran G. Amyloidogenic processing of beta-amyloid precursor protein in intracellular compartments. Neurology. 2006; 66:2 Suppl 1. S69–S73.7. Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, et al. Amyloid-beta: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-beta. Brain Res Brain Res Rev. 2003; 43:1–16.
Article8. Muresan V, Ladescu Muresan Z. Amyloid-β precursor protein: multiple fragments, numerous transport routes and mechanisms. Exp Cell Res. 2015; 334:45–53.
Article9. Cappai R. Making sense of the amyloid precursor protein: its tail tells an interesting tale. J Neurochem. 2014; 130:325–327.
Article10. Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008; 283:29615–29619.
Article11. Alban A, David SO, Bjorkesten L, Andersson C, Sloge E, Lewis S, et al. A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003; 3:36–44.
Article12. Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002; 1:845–867.
Article13. Park CH, Kang JS, Yoon EH, Shim JW, Suh-Kim H, Lee SH. Proneural bHLH neurogenin 2 differentially regulates Nurr1-induced dopamine neuron differentiation in rat and mouse neural precursor cells in vitro. FEBS Lett. 2008; 582:537–542.
Article14. Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996; 10:3129–3140.
Article15. Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002; 277:32046–32053.
Article16. Noh MY, Koh SH, Kim Y, Kim HY, Cho GW, Kim SH. Neuroprotective effects of donepezil through inhibition of GSK-3 activity in amyloid-beta-induced neuronal cell death. J Neurochem. 2009; 108:1116–1125.
Article17. Park HH, Yu HJ, Kim S, Kim G, Choi NY, Lee EH, et al. Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, through scavenging free radicals and enhancing survival signals. Neurotoxicology. 2016; 55:131–141.
Article18. Lee KY, Koh SH, Noh MY, Kim SH, Lee YJ. Phosphatidylinositol-3-kinase activation blocks amyloid beta-induced neurotoxicity. Toxicology. 2008; 243:43–50.
Article19. Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, Ameneiros-Rodríguez E, Donapetry-García C, Vila-Altesor M, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014; 17:76–100.
Article20. Pineda JR, Callender R, Schwartz SD. Ligand binding and protein dynamics in lactate dehydrogenase. Biophys J. 2007; 93:1474–1483.
Article21. Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004; 101:2173–2178.
Article22. Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol. 2012; 45:499–506.
Article23. Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007; 104:13798–13803.
Article24. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002; 418:191–195.
Article25. Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006; 129(Pt 11):3006–3019.
Article26. Fujita K, Motoki K, Tagawa K, Chen X, Hama H, Nakajima K, et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer's disease. Sci Rep. 2016; 6:31895.
Article27. Arakaki TL, Pezza JA, Cronin MA, Hopkins CE, Zimmer DB, Tolan DR, et al. Structure of human brain fructose 1,6-(bis)phosphate aldolase: linking isozyme structure with function. Protein Sci. 2004; 13:3077–3084.
Article28. Sekar Y, Moon TC, Slupsky CM, Befus AD. Protein tyrosine nitration of aldolase in mast cells: a plausible pathway in nitric oxide-mediated regulation of mast cell function. J Immunol. 2010; 185:578–587.
Article29. Osawa H, Smith CA, Ra YS, Kongkham P, Rutka JT. The role of the membrane cytoskeleton cross-linker ezrin in medulloblastoma cells. Neuro Oncol. 2009; 11:381–393.
Article30. Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993; 105(Pt 4):1025–1043.
Article31. Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr Opin Cell Biol. 1999; 11:109–116.
Article32. Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, et al. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007; 178:1938–1947.
Article33. Johnson MW, Miyata H, Vinters HV. Ezrin and moesin expression within the developing human cerebrum and tuberous sclerosis-associated cortical tubers. Acta Neuropathol. 2002; 104:188–196.
Article34. Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J Neurochem. 2004; 91:451–461.
Article35. Pap M, Cooper GM. Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta signaling pathway. Mol Cell Biol. 2002; 22:578–586.
Article36. Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001; 7:1321–1327.
Article37. Liu S, Liu S, Wang X, Zhou J, Cao Y, Wang F, et al. The PI3K-Akt pathway inhibits senescence and promotes self-renewal of human skin-derived precursors in vitro. Aging Cell. 2011; 10:661–674.
Article38. Choi H, Park HH, Koh SH, Choi NY, Yu HJ, Park J, et al. Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology. 2012; 33:85–90.
Article39. Choi H, Park HH, Lee KY, Choi NY, Yu HJ, Lee YJ, et al. Coenzyme Q10 restores amyloid beta-inhibited proliferation of neural stem cells by activating the PI3K pathway. Stem Cells Dev. 2013; 22:2112–2120.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amyloid-beta oligomers regulate the properties of human neural stem cells through GSK-3beta signaling
- The Effects of Psychotropic Drugs on the beta-Amyloid-Induced Cytotoxicity in PC12 Cells
- Inhibition of Amyloid beta Peptide-induced Neuronal Cytotoxicity by EGCG
- Protective Effect of Fibroin BF-7 on Neuronal Cell Death in Alzheimer Model using Amyloid beta Peptide
- Neuroprotective Effects of EGCG against Amyloid-beta (1-42)-Induced Apoptosis