J Korean Soc Spine Surg.
2019 Mar;26(1):11-20. 10.4184/jkss.2019.26.1.11.
Comparison of the Efficacy and Safety Profiles of a Mixed ‘PF-72’ and ‘0.75% Ropivacaine HCl’ Versus a ‘0.75% Ropivacaine HCl’ and No Treatment Group: A Randomized, Single-Blind, Single-Institution Pilot Study
- Affiliations
-
- 1Department of Orthopedic Surgery, College of Medicine, Soonchunhyang University Seoul Hospital, Seoul, Republic of Korea. schsbj@gmail.com
- 2Department of Orthopedic Surgery, College of Medicine, Soonchunhyang University Bucheon Hospital, Seoul, Republic of Korea.
- 3TGel Bio, Co, LTD, Seoul, Republic of Korea.
- KMID: 2442343
- DOI: http://doi.org/10.4184/jkss.2019.26.1.11
Abstract
- STUDY DESIGN: Prospective pilot study
OBJECTIVES
The efficacy and safety of "˜PF-72' for management of postoperative acute pain through a mixed "˜PF-72' and 0.75% ropivacaine hydrochloride solution in patients with posterior spine surgery was evaluated as "˜0.75% ropivacaine' and "˜untreated' controls. SUMMARY OF LITERATURE REVIEW: Postoperative acute pain is major surgical side effect that lead to the deterioration of the quality of life. Traditional pain control results in variable side effects, and multimodal pain management has been recommended as an alternative. Local anesthetics is a short-acting time lower than 12 hours. There is controversy about the efficiency and stability of thermoreactive hydrogel products as a drug delivery system.
MATERIALS AND METHODS
Patients scheduled for posterior spine surgery were enrolled by the inclusion criteria. In the treated group, PF-72 and ropivacaine mixture was injected to the surgical wound before closure. In control group 1, only 0.75% ropivacaine hydrochloride was injected. In the control group 2, the surgical site was without injection. Ten patients were randomly assigned to each group and standardized drugs for pain control were applied postoperatively and rescue regimens were applied when necessary. Postoperative pain score and the cumulative area under the curve (AUC) of pain score were compared. The percentage of subjects who were painless (pain score ≤ 3) was examined at each observation point. The first time of injection and the total dose of the rescue regimen were examined. Postoperative nausea and vomiting (PONV) were also evaluated.
RESULTS
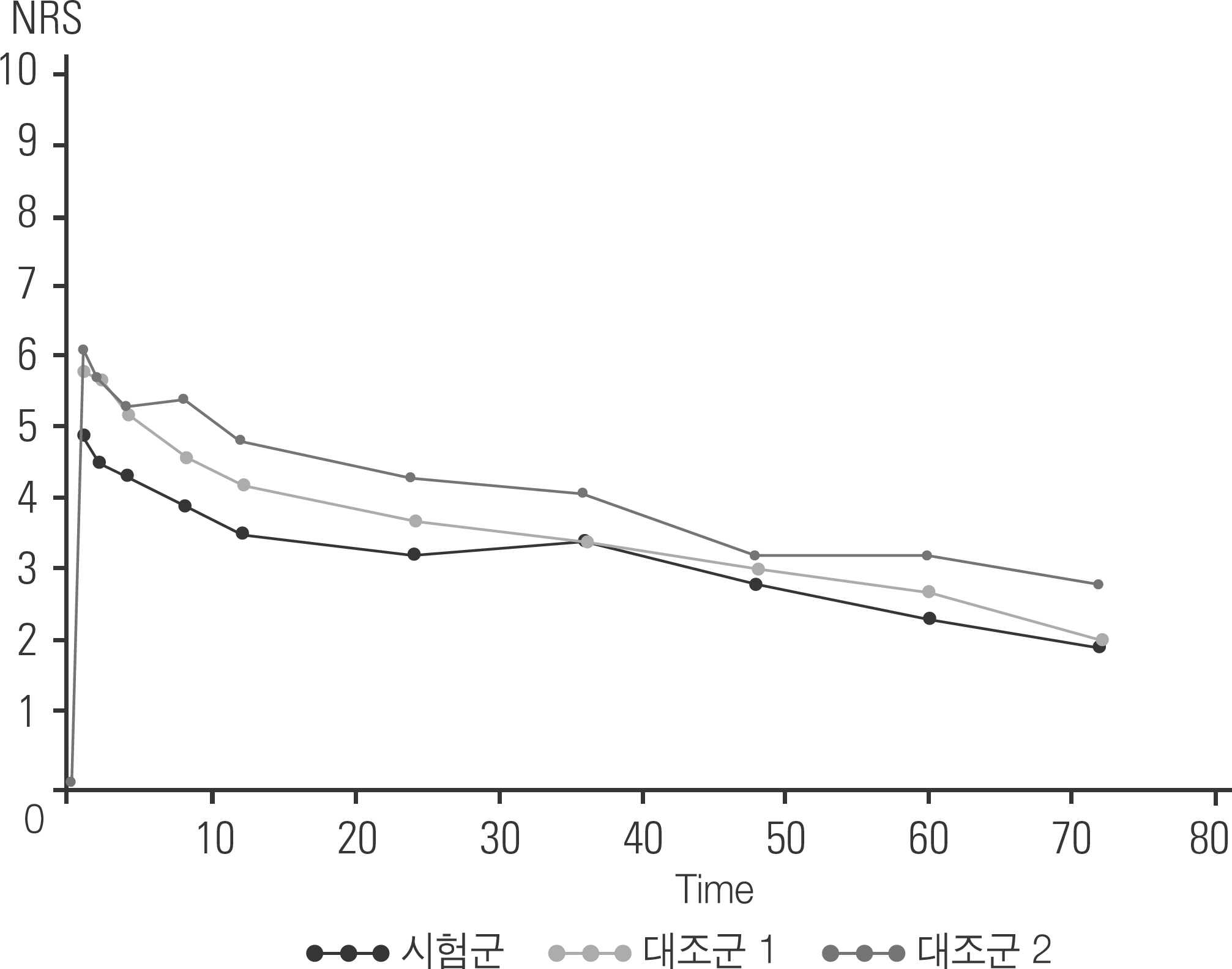
There was no significant difference in demographic data. The sum AUC of pain scores in the treated group was significantly lower than that in the control group 1 and 2 at all observation times. The proportion of painless patients was significantly higher in the treated group than in the control group 2. There was no significant difference between the first administration time and the total usage of the rescue regimen, and the percentage of patients with PONV at all time points. There was no statistically significant difference in the incidence of adverse events.
CONCLUSIONS
PF-72 and ropivacaine mixture showed significant effects for pain management up to 72 hours postoperatively for the patients who underwent posterior spinal surgery without fatal complications.
MeSH Terms
Figure
Reference
-
1. Chung F. Recovery pattern and home-readiness after am-bulatory surgery. Anesth Analg. 1995 May; 80(5):896–902. DOI: DOI:10.1097/00000539-199505000-00008.
Article2. Charles E. Argoff. Recent Management Advances in Acute Postoperative Pain. World Institute of Pain. 2014; 14(5):477–87. DOI: 10.1111/papr.12108.3. Fortier J, Chung F, Su J. Unanticipated admission after am-bulatory surgery - a prospective study. Can J Anaesth. 1998 Jul; 45(7):612–9. DOI: 10.1007/BF03012088.
Article4. Sutters KA, Miaskowski C. Inadequate pain management and associated morbidity in children at home after tonsil-lectomy. J Pediatr Nurs. 1997 Jun; 12(3):178–85. DOI: 10.1016/s0882-5963 (97)80075-9.
Article5. Ahn EK, Kim JH, Chon SS, at al. The Comparison of Ropivacaine and Bupivacaine in Epidural Patient Controlled Analgesia (PCA). Korean Journal of Anesthesiology. 2002; 42(5):646–51. DOI: 10.4097/kjae.2002.42.5.646.6. Park YS, Kim YC, Kim YH, at al. The Effects of Postoperative Patient Controlled Analgesia after Spinal Fusion. J Korean Soc Spine Surg. 1999 May; 6(1):141–5. DOI: 10.4184/jkss.1999.6.1.141.7. Schenk MR, Putzier M, Kü gler B, at al. Postoperative analgesia after major spine surgery: patient-controlled epidural analgesia versus patient-controlled intravenous analgesia. Anesth Analg. 2006 Nov; 103(5):1311–7. DOI: 10.1213/01.ane/0000247966.49492.72.8. Bianconi M, Ferraro L, Traina GC, at al. Pharmacokinet-ics and efficacy of ropivacaine continuous wound instil-lation after joint replacement surgery. Br J Anaesth. 2003 Dec; 91(6):830–5. DOI: 10.1093/bja/aeg277.9. Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013 Sep; 26(3):191–6. DOI: 10.1055/s-0033-1351138.
Article10. Vinson-Bonnet B, Coltat JC, Fingerhut A, at al. Local Infiltration with Ropivacaine Improves Immediate Postoperative Pain Control After Hemorrhoidal Surgery. Dis Colon Rec-tum. 2002 Jan; 45(1):104–8. DOI: 10.1007/s10350-004-6121-4.11. Wang Z, Huang H, Yang S, at al. Long-term effect of ropivacaine nanoparticles for sciatic nerve block on postoperative pain in rats. Int J Nanomedicine. 2016 May 17; 11:2081–90. DOI: 10.2147/ijn.s101563.12. Kim T, Seol DR, Hahm SC, at al. Analgesic effect of intra-articular injection of temperature-responsive hydrogel con-taining bupivacaine on osteoarthritic pain in rats. Biomed Res Int. 2015; 2015:812949. DOI: 10.1155/2015/812949.13. Choi DH. Anesthesia and Pain Medicine. Korean Society for Intravenous Anesthesia. 2004 Dec; 8(4):193–6.14. Forst J, Wolff S, Thamm P, at al. Pain therapy following joint replacement. A randomized study of patient-controlled analgesia versus conventional pain therapy. Arch Orthop Trauma Surg. 1999; 119(5-6):267–70. DOI: 10.1007/s004020050407.15. Fisher CG, Belanger L, Gofton EG, at al. Prospective randomized clinical trial comparing patient-controlled intravenous analgesia with patient controlled epidural analgesia after lumbar spinal fusion. Spine (Phila Pa 1976). 2003 Apr 15; 28(8):739–43. DOI: 10.1097/01. brs.0000058943.93281.28.16. Rathmell JP, Pino CA, Taylor R, at al. Intrathecal mor-phine for postoperative analgesia: a randomized, con-trolled, dose-ranging study after hip and knee arthroplasty. Anesth Analg. 2003 Nov; 97(5):1452–7. DOI: 10.1213/01. ane.0000083374.44039.9e.17. Lambrechts M, O'Brien MJ, Savoie FH, at al. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013 Sep 6; 7:885–90. DOI: 10.2147/ppa.s32175.18. Cohen SM. Extended pain relief trial utilizing infiltration of Exparel®, a long-acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012; 5:567–72. DOI: 10.2147/jpr.s38621.19. Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012; 5:257–64. DOI: 10.2147/jpr.s27894.20. Nguyen QV, Huynh DP, Park JH, at al. Injectable poly-meric hydrogels for the delivery of therapeutic agents: A review. European Polymer Journal. 2015; 72:602–19. DOI: 10.1016/j.eurpolymj.2015.03.016.21. Hoare TR, Kohane DS. Hydrogels in drug delivery: Prog-ress and challenges. Polymer. 2008 Apr; 49(8):1993–2007. DOI: 10.1016/j.polymer.2008.01.027.
Article22. Oh KS, Hwang CS, Lee HY, et al. Preclinical studies of ropivacaine extended-release from a temprature responsive hydrogel for prolonged relief of pain at the surgical wound. Int J Pharm. 2019 Mar 10; 558:225–30. DOI: 10.1016/j.ijpharm.2019.01.011.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of clinical efficacy of ropivacaine and lignocaine with adrenaline for implant surgery anesthesia: a split-mouth randomized controlled clinical trial
- Clinical efficacy of 0.75% ropivacaine vs. 2% lignocaine hydrochloride with adrenaline (1:80,000) in patients undergoing removal of bilateral maxillary third molars: a randomized controlled trial
- A comparison of the onset time of complete blockade of the sciatic nerve in the application of ropivacaine and its equal volumes mixture with lidocaine: a double-blind randomized study
- Comparison of Sensory and Motor Nerve Block in Epidural Anesthesia Using a Different Concentrations of Ropivacaine for Cesarean Section
- Analgesia after Arthroscopic Shoulder Surgery: Study of Effective Concentration of Continuous Patient-Controlled Subacromial Ropivacaine Infusion