Cancer Res Treat.
2019 Jan;51(1):313-325. 10.4143/crt.2018.105.
Diagnostic Significance of p38 Isoforms (p38α, p38β, p38γ, p38δ) in Head and Neck Squamous Cell Carcinoma: Comparative Serum Level Evaluation and Design of Novel Peptide Inhibitor Targeting the Same
- Affiliations
-
- 1Department of Biophysics, All India Institute of Medical Sciences, Ansari Nagar, India. sharmistha_d@hotmail.com
- 2School of Computational and Integrative Sciences, Jawaharlal Nehru University, New Delhi, India.
- 3Department of Radiation Oncology, All India Institute of Medical Sciences, Ansari Nagar, India.
- KMID: 2437622
- DOI: http://doi.org/10.4143/crt.2018.105
Abstract
- PURPOSE
The p38 mitogen-activated protein kinase (MAPKs) play a crucial role in the production of pro-inflammatory cytokines and over-expression of it increase cytokines which promote cancer. Among four isoforms, p38α has been well studied in head and neck squamous cell carcinoma (HNSCC) and other cancers as a therapeutic target. p38δ has recently emerged as a potential disease-specific drug target. Elevated serum p38α level in HNSCC was reported earlier from our lab. This study aims to estimate the levels of p38 MAPK-isoforms in the serum of HNSCC and design peptide inhibitor targeting the same.
MATERIALS AND METHODS
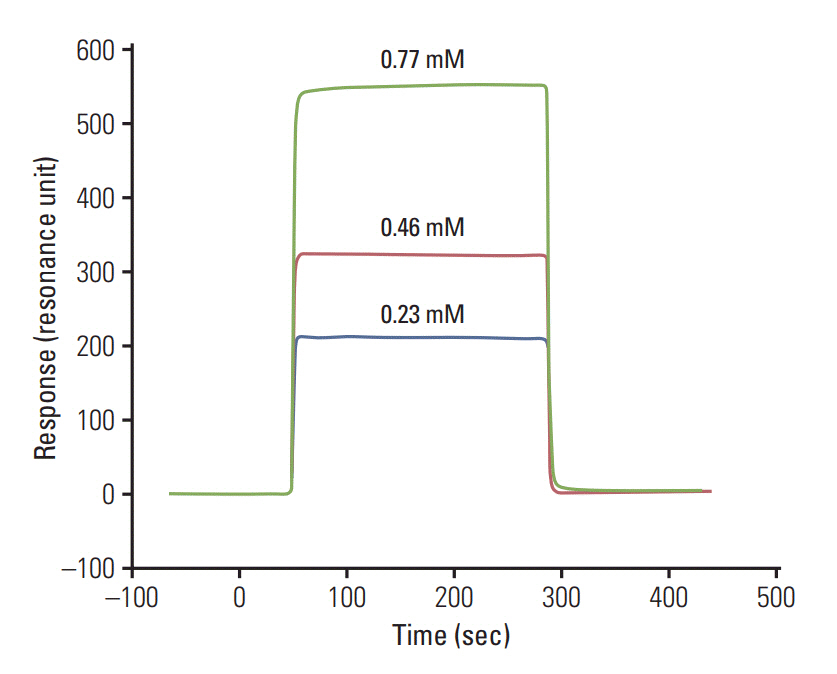
Levels of p38 MAPK isoforms in the serum of HNSCC and healthy controls were quantified by surface plasmon resonance technology. The peptide inhibitor for p38 MAPK was designed by molecular modeling using Grid-based Ligand Docking with Energetics tools and compared with known specific inhibitors.
RESULTS
We have observed highly elevated levels of all four isoforms of p38 MAPK in serum of HNSCC patients compared to the control group. Further, serum p38α, p38β, and p38δ levels were down regulated after therapy in follow-up patients, while p38γ showed no response to the therapy. Present study screened designed peptide WFYH as a specific inhibitor against p38δ. The specific inhibitor of p38δ was found to have no effect on p38α due to great structural difference at ATP binding pocket.
CONCLUSION
In this study, first time estimated the levels of p38 MAPK isoforms in the serum of HNSCC. It can be concluded that p38 MAPK isoforms can be a diagnostic and prognostic marker for HNSCC and p38δ as a therapeutic target.
Keyword
MeSH Terms
-
Adenosine Triphosphate
Carcinoma, Squamous Cell*
Cytokines
Epithelial Cells*
Follow-Up Studies
Head*
Humans
Models, Molecular
Neck*
p38 Mitogen-Activated Protein Kinases
Protein Isoforms*
Protein Kinases
Surface Plasmon Resonance
Adenosine Triphosphate
Cytokines
Protein Isoforms
Protein Kinases
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
References
1. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24:2137–50.
Article2. Risco A, Cuenda A. New insights into the p38gamma and p38delta MAPK pathways. J Signal Transduct. 2012; 2012:520289.3. Gill K, Mohanti BK, Ashraf MS, Singh AK, Dey S. Quantification of p38alphaMAP kinase: a prognostic marker in HNSCC with respect to radiation therapy. Clin Chim Acta. 2012; 413:219–25.4. Gill K, Singh AK, Kapoor V, Nigam L, Kumar R, Holla P, et al. Development of peptide inhibitor as a therapeutic agent against head and neck squamous cell carcinoma (HNSCC) targeting p38alpha MAP kinase. Biochim Biophys Acta. 2013; 1830:2763–9.5. Gill K, Nigam L, Singh R, Kumar S, Subbarao N, Chauhan SS, et al. The rational design of specific peptide inhibitor against p38alpha MAPK at allosteric-site: a therapeutic modality for HNSCC. PLoS One. 2014; 9:e101525.6. Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004; 47:1750–9.
Article7. Singh AK, Pandey R, Gill K, Singh R, Saraya A, Chauhan SS, et al. p38beta MAP kinase as a therapeutic target for pancreatic cancer. Chem Biol Drug Des. 2012; 80:266–73.8. Hawkins J, Zheng S, Frantz B, LoGrasso P. p38 map kinase substrate specificity differs greatly for protein and peptide substrates. Arch Biochem Biophys. 2000; 382:310–3.
Article9. Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogenactivated protein kinase inhibitors: mechanisms and therapeutic potentials. Pharmacol Ther. 1999; 82:389–97.
Article10. Yurtsever Z, Scheaffer SM, Romero AG, Holtzman MJ, Brett TJ. The crystal structure of phosphorylated MAPK13 reveals common structural features and differences in p38 MAPK family activation. Acta Crystallogr D Biol Crystallogr. 2015; 71(Pt 4):790–9.
Article11. Bellon S, Fitzgibbon MJ, Fox T, Hsiao HM, Wilson KP. The structure of phosphorylated p38gamma is monomeric and reveals a conserved activation-loop conformation. Structure. 1999; 7:1057–65.12. Dougherty DA. Cation-pi interactions in chemistry and biology: a new view of benzene, Phe, Tyr, and Trp. Science. 1996; 271:163–8.13. Fitzgerald CE, Patel SB, Becker JW, Cameron PM, Zaller D, Pikounis VB, et al. Structural basis for p38alpha MAP kinase quinazolinone and pyridol-pyrimidine inhibitor specificity. Nat Struct Biol. 2003; 10:764–9.14. Enslen H, Brancho DM, Davis RJ. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 2000; 19:1301–11.
Article15. Azevedo R, van Zeeland M, Raaijmakers H, Kazemier B, de Vlieg J, Oubrie A. X-ray structure of p38α bound to TAK-715: comparison with three classic inhibitors. Acta Crystallogr D Biol Crystallogr. 2012; 68(Pt 8):1041–50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- EGFR-targeted Therapy in Head and Neck Squamous Cell Carcinoma
- Herpes Viral Gene Therapy for the Treatment of Head and Neck Squamous Cell Carcinoma
- Serum soluble interleukin-2 receptor and neoptrin in patients with head and neck squamous cell carcinoma
- Clinical analysis of distant metastases in the squamous cell carcinoma of head and neck
- The Relationship between Expression of E-Cadherin, Clinical Staging and Differentiation in Squamous Cell Carcinomas of the Head and Neck