J Vet Sci.
2019 Jan;20(1):51-57. 10.4142/jvs.2019.20.1.51.
Novel application of Influenza A virus-inoculated chorioallantoic membrane to characterize a NP-specific monoclonal antibody for immunohistochemistry assaying
- Affiliations
-
- 1Department of Epidemiology, Animal Health Research Institute, Council of Agriculture, New Taipei City 25158, Taiwan.
- 2Department of Veterinary Medicine, School of Veterinary Medicine, National Taiwan University, Taipei 10617, Taiwan. ivancheng@ntu.edu.tw
- 3Department of Veterinary Medicine, College of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung 91201, Taiwan.
- KMID: 2434777
- DOI: http://doi.org/10.4142/jvs.2019.20.1.51
Abstract
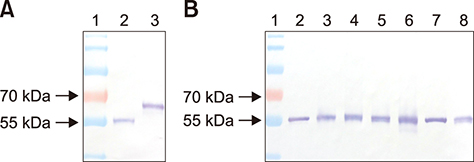
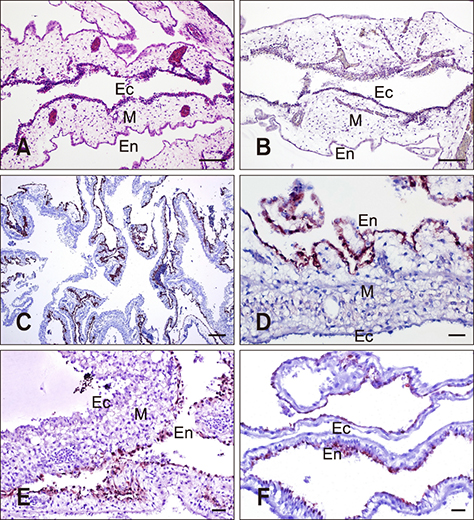
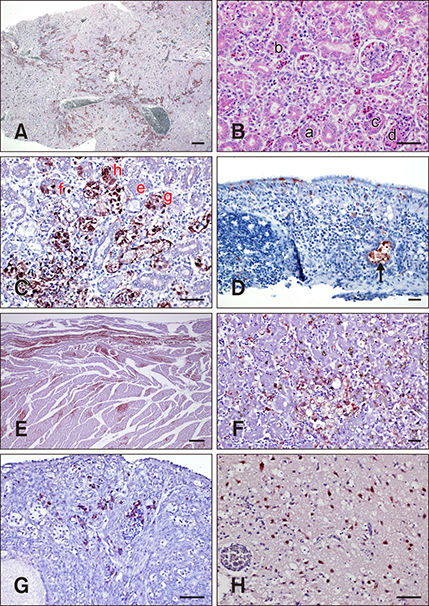
- Monoclonal antibodies (MAbs) are widely applied in disease diagnoses. Herein, we report a MAb, WF-4, against Influenza A virus nucleoprotein (NP), its broad response with Influenza A virus, and its application in an immunohistochemistry (IHC) assay. WF-4 was screened by immunofluorescence assay (IFA). The results showed that its reactivity with baculovirus-expressed full-length recombinant NP (rNP) in Western blot (WB), indicating its IHC applicability. Fifteen Influenza A virus (reference subtypes H1 to H15) infected chicken embryonated chorioallantoic membranes (CAM), fixed by formalin, were all detectable in the WF-4-based IHC assay. Also, the reactivity of the IHC test with NP from experimentally inoculated H6N1 and from all recent outbreaks of H5 subtype avian Influenza A virus (AIV) field cases in Taiwan showed positive results. Our data indicate that CAM, a by-product of Influenza A virus preparation, is helpful for Influenza A virus-specific MAb characterization, and that the WF-4 MAb recognizes conserved and linear epitopes of Influenza A virus NP. Therefore, WF-4 is capable of detecting NP antigens via IHC and may be suitable for developing various tests for diagnosis of Influenza A virus and, especially, AIV infection.
Keyword
MeSH Terms
-
Animals
Antibodies, Monoclonal
Blotting, Western
Chickens
Chorioallantoic Membrane*
Diagnosis
Disease Outbreaks
Epitopes
Fluorescent Antibody Technique
Formaldehyde
Immunohistochemistry*
Influenza A virus
Influenza in Birds
Influenza, Human*
Nucleoproteins
Taiwan
Antibodies, Monoclonal
Epitopes
Formaldehyde
Nucleoproteins
Figure
Reference
-
1. Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol. 2000; 74:3–13.
Article2. Alexander DJ. Orthomyxoviridae: avian influenza. In : Jordan FTW, Pattison M, Alexander DJ, Faragher T, editors. Poultry Diseases. 5th ed. London: W.B. Saunders;2002. p. 281–290.3. Beard CW. Demonstration of type-specific influenza antibody in mammalian and avian sera by immunodiffusion. Bull World Health Organ. 1970; 42:779–785.4. Cheng MC, Soda K, Lee MS, Lee SH, Sakoda Y, Kida H, Wang CH. Isolation and characterization of potentially pathogenic H5N2 influenza virus from a chicken in Taiwan in 2008. Avian Dis. 2010; 54:885–893.
Article5. Coons AH, Kaplan MH. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950; 91:1–13.6. Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005; 79:2814–2822.
Article7. Halvorson DA, Karunakaran D, Newman JA. Avian influenza in caged laying chickens. Avian Dis. 1980; 24:288–294.
Article8. Harlow E, Lane D. Monoclonal antibodies. In : Harlow E, Lane D, editors. Antibodies: A Laboratory Manual. New York: Cold Spring Harbor Laboratory;1988. p. 139–244.9. Hong TH, Chen ST, Tang TK, Wang SC, Chang TH. The production of polyclonal and monoclonal antibodies in mice using novel immunization methods. J Immunol Methods. 1989; 120:151–157.
Article10. Huang PY, Lee CD, Yip CH, Cheung CL, Yu G, Lam TT, Smith DK, Zhu H, Guan Y. Genetic characterization of highly pathogenic H5 influenza viruses from poultry in Taiwan, 2015. Infect Genet Evol. 2016; 38:96–100.
Article11. Lamb RL, Krug RM. Orthomyxoviridae: the viruses and their replication. In : Fields BN, Knipe DM, editors. Fields Virology. 1:4th ed. Philadelphia: Lippincott Williams & Wilkins;2001. p. 1216–1253.12. Lee CCD, Zhu H, Huang PY, Peng L, Chang YC, Yip CH, Li YT, Cheung CL, Compans R, Yang C, Smith DK, Lam TTY, King CC, Guan Y. Emergence and evolution of avian H5N2 influenza viruses in chickens in Taiwan. J Virol. 2014; 88:5677–5686.
Article13. Lee MS, Chen LH, Chen YP, Liu YP, Li WC, Lin YL, Lee F. Highly pathogenic avian influenza viruses H5N2, H5N3, and H5N8 in Taiwan in 2015. Vet Microbiol. 2016; 187:50–57.
Article14. Slemons RD, Swayne DE. Replication of a waterfowl-origin influenza virus in the kidney and intestine of chickens. Avian Dis. 1990; 34:277–284.
Article15. Soda K, Cheng MC, Yoshida H, Endo M, Lee SH, Okamatsu M, Sakoda Y, Wang CH, Kida H. A low pathogenic H5N2 influenza virus isolated in Taiwan acquired high pathogenicity by consecutive passages in chickens. J Vet Med Sci. 2011; 73:767–772.
Article16. Swayne DE, Suarez DL. Highly pathogenic avian influenza. Rev Sci Tech. 2000; 19:463–482.
Article17. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–179.
Article18. Webster RG, Hulse DJ. Microbial adaptation and change: avian influenza. Rev Sci Tech. 2004; 23:453–465.
Article19. World Organisation for Animal Health (OIE). Avian influenza (infection with avian influenza viruses). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 2.3.4. Paris: OIE;2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Universal Approach to Getting Ahead for Influenza B Vaccines
- Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus
- The Morphological Changes by the Time of Administration of Zanamivir in Rabbit Nasal Mucosa Infected with Influenza A Virus
- Vaccine Strategy That Enhances the Protective Efficacy of Systemic Immunization by Establishing LungResident Memory CD8 T Cells Against Influenza Infection
- Preparation and immunogenicity of influenza virus-like particles using nitrocellulose membrane filtration