Clin Exp Vaccine Res.
2017 Jan;6(1):61-66. 10.7774/cevr.2017.6.1.61.
Preparation and immunogenicity of influenza virus-like particles using nitrocellulose membrane filtration
- Affiliations
-
- 1Department of Global Medical Science, Sungshin University, Seoul, Korea. jmsong@sungshin.ac.kr
- KMID: 2366893
- DOI: http://doi.org/10.7774/cevr.2017.6.1.61
Abstract
- PURPOSE
Nitrocellulose membrane-based filtration system (NCFS) is widely used for protein concentration. In this study, we applied NCFS for production of virus-like particle (VLP) as a vaccine candidate and evaluated yield property and immunogenicity.
MATERIALS AND METHODS
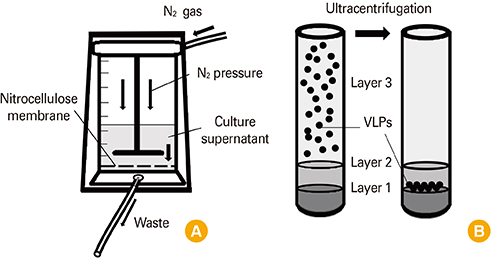
Influenza VLPs were generated by baculovirus-insect cell protein expression system. NCFS and sucrose gradient ultracentrifugation were used for purification of VLP. Immunogenicity of VLP was evaluated by animal experiment.
RESULTS
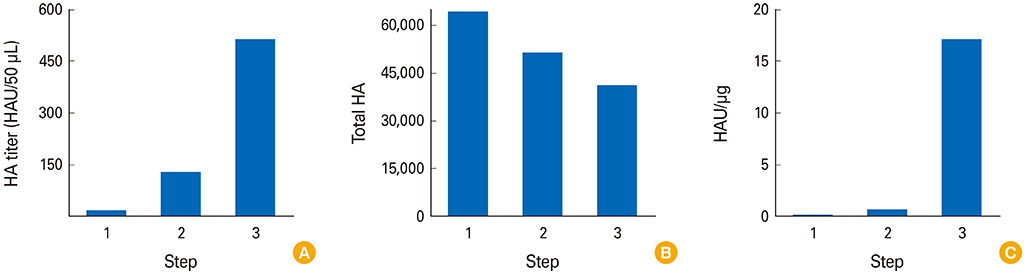
Influenza VLPs expressing hemagglutinin (HA) and neuraminidase proteins derived from highly pathogenic influenza virus (H5N8) were effectively produced and purified by NCFS. HA activity of VLP which correlated with antigenicity was well conserved during multiple purification steps. This NCFS based purified VLPs induced influenza virus-specific antibody responses.
CONCLUSION
Our results indicate that the influenza VLP vaccine could be prepared by NCFS without loss of immunogenicity and elicit antigen-specific immune responses.
MeSH Terms
Figure
Reference
-
1. de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL. A pandemic warning? Nature. 1997; 389:554.
Article2. Wu H, Peng X, Xu L, et al. Novel reassortant influenza A (H5N8) viruses in domestic ducks, eastern China. Emerg Infect Dis. 2014; 20:1315–1318.
Article3. Jeong J, Kang HM, Lee EK, et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet Microbiol. 2014; 173:249–257.
Article4. Park SJ, Si YJ, Kim J, et al. Cross-protective efficacies of highly-pathogenic avian influenza H5N1 vaccines against a recent H5N8 virus. Virology. 2016; 498:36–43.
Article5. Zeng X, Chen P, Liu L, et al. Protective efficacy of an H5N1 inactivated vaccine against challenge with lethal H5N1, H5N2, H5N6, and H5N8 influenza viruses in chickens. Avian Dis. 2016; 60:1 Suppl. 253–255.
Article6. Steensels M, Rauw F, van den Berg T, et al. Protection afforded by a recombinant Turkey herpesvirus-H5 vaccine against the 2014 European highly pathogenic H5N8 avian influenza strain. Avian Dis. 2016; 60:1 Suppl. 202–209.
Article7. Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015; 14:167–182.
Article8. Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ. 2009; 339:b3680.
Article9. Chroboczek J, Szurgot I, Szolajska E. Virus-like particles as vaccine. Acta Biochim Pol. 2014; 61:531–539.
Article10. Thompson CM, Petiot E, Lennaertz A, Henry O, Kamen AA. Analytical technologies for influenza virus-like particle candidate vaccines: challenges and emerging approaches. Virol J. 2013; 10:141.
Article11. Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010; 9:1149–1176.
Article12. Singh M, Chakrapani A, O'Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007; 6:797–808.
Article13. Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005; 23:5751–5759.
Article14. Krauss S, Stallknecht DE, Slemons RD, et al. The enigma of the apparent disappearance of Eurasian highly pathogenic H5 clade 2.3.4.4 influenza A viruses in North American waterfowl. Proc Natl Acad Sci U S A. 2016; 113:9033–9038.15. Lee DH, Sharshov K, Swayne DE, et al. Novel reassortant clade 2.3.4.4 avian influenza A(H5N8) virus in wild aquatic birds, Russia, 2016. Emerg Infect Dis. 2017; 23:359–360.16. Verhagen JH, Herfst S, Fouchier RA. Infectious disease. How a virus travels the world. Science. 2015; 347:616–617.
Article17. Ren Z, Ji X, Meng L, et al. H5N1 influenza virus-like particle vaccine protects mice from heterologous virus challenge better than whole inactivated virus. Virus Res. 2015; 200:9–18.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Progress and hurdles in the development of influenza virus-like particle vaccines for veterinary use
- Influence of temperature on the antigenic changes of virus-like particles
- Prevention and Treatment of Influenza
- Benign Acute Childhood Myositis Associated with Influenza B Virus
- Clinical Use of Tamiflu (Oseltamivir)