Nat Prod Sci.
2018 Dec;24(4):241-246. 10.20307/nps.2018.24.4.241.
Identification and HPLC Quantification of a Phytoecdysone and Three Phenolic Glycosides in Lamium takesimense Nakai
- Affiliations
-
- 1Department of Agro-industrial Technology, LambungMangkurat University, Banjarbaru 70714, Indonesia.
- 2Deparment of Oriental Medicine, Sangji University, Wonju 26339, Korea.
- 3Department of Pharmaceutical Engineering, Sangji University, Wonju 26339, Korea. hjpark@sangji.ac.kr
- KMID: 2432418
- DOI: http://doi.org/10.20307/nps.2018.24.4.241
Abstract
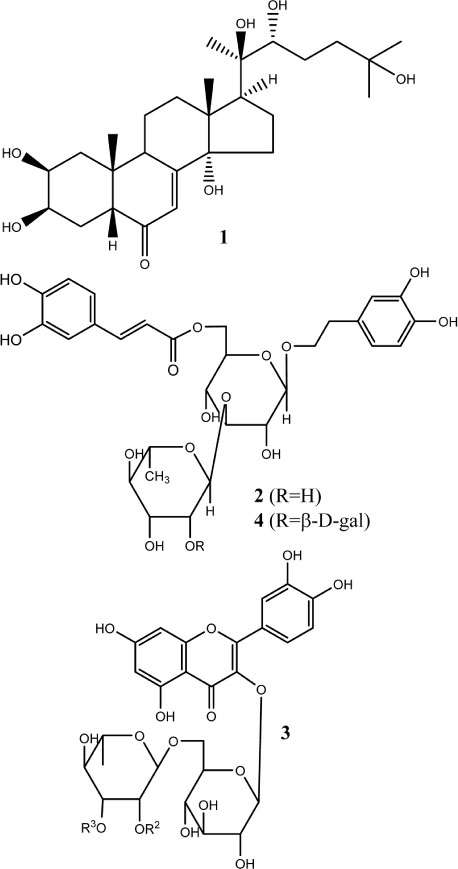
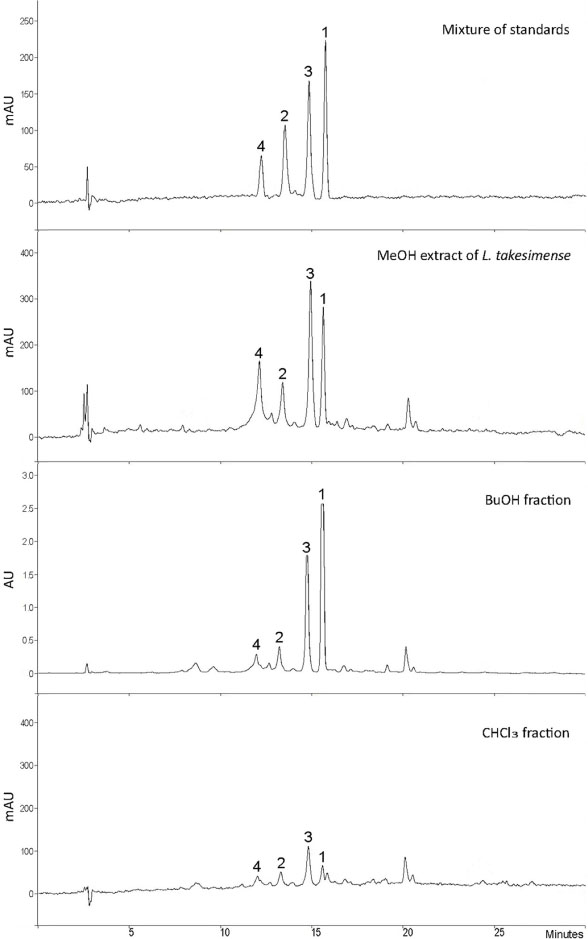
- The herbs of Lamium takesimense Nakai (Lamiaceae) is used to treat spasmodic and inflammatory disease. The four polar compounds, ecdysterone, isoacteoside, rutin and lamiuside C, were isolated and identified from the BuOH fraction of the L. takesimense MeOH extract. HPLC quantification was performed on a Capcell Pak C18 column (5 µm, 4.6 mm × 250 mm) with a gradient elution of Hâ‚‚O and 0.05% acetic acid in MeOH. The HPLC method was validated in terms of linearity, sensitivity, stability, precision, and accuracy. The quantitative level in plant material was determined as the following order: lamiuside C (4, 3.75 mg/g dry weight) > ecdysterone (1, 1.93 mg/g) > isoacteoside (2, 1.32 mg/g) > rutin (3, 0.97 mg/g).
Keyword
MeSH Terms
Figure
Reference
-
1. Dinan L. Phytochemistry. 2014; 57:325–339.2. Wang T, Zhang X, Xie W. Am J Chin Med. 2012; 40:1123–1141.3. Kurisu M, Miyamae Y, Murakami K, Han J, Isoda H, Irie K, Shigemori H. Biosci Biotechnol Biochem. 2013; 77:1329–1332.4. Lee CB. Colored Flora of Korea. Seoul: Hyangmunsa;2014. p. 129.5. Ito N, Nihei T, Kakuda R, Yaoita Y, Kikuchi M. Chem Pharm Bull. 2006; 54:1705–1708.6. Agzmova MA, Isaev IMO, Mamathanov AU, Isaev MIO, Ibragimov TF. Adv Biol Chem. 2014; 4:1–4.7. Schlauer J, Budzianowski J, Kukulczanka K, Ratajczak L. Acta Soc Bot Pol. 2004; 73:9–15.8. Park HW, Baek NI, Kim SH, Kwon BM, Chung IS, Park MH, Kim SH, Kim DK. Korean J Pharmacogn. 2004; 35:320–323.9. Báthori M, Tóth N, Hunyadi A, Márki A, Zádor E. Curr Med Chem. 2008; 15:75–91.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phenolic Compounds Isolated from Opuntia ficus-indica Fruits
- Flavonoid Glycosides from the Flowers of Pulsatilla koreana Nakai
- HPLC and GC-MS Analysis of Phenolic Substances in Acer tegmentosum
- Preparation and Evaluation of Anti-Emetic Ginger Orodispersible Tablets from Standardized Extract using Phenolic Profile Effects
- Analysis of Phenolic Acid Content and Antioxidant Activity of Chestnut Honey from Different Regions of Korea