Nat Prod Sci.
2018 Dec;24(4):229-234. 10.20307/nps.2018.24.4.229.
Quantitative Analysis of Dammarane-type Ginsenosides in Different Ginseng Products
- Affiliations
-
- 1Department of Integrative Plant Science, Chung-Ang University, Anseong 17546, Republic of Korea. hykim@gntech.ac.kr
- 2Department of Biology, University of San Carlos, Cebu 6000, Philippines.
- 3Department of Food Science, Gyeongnam National University of Science and Technology, Jinju 52725, Republic of Korea. slee@cau.ac.kr
- KMID: 2432416
- DOI: http://doi.org/10.20307/nps.2018.24.4.229
Abstract
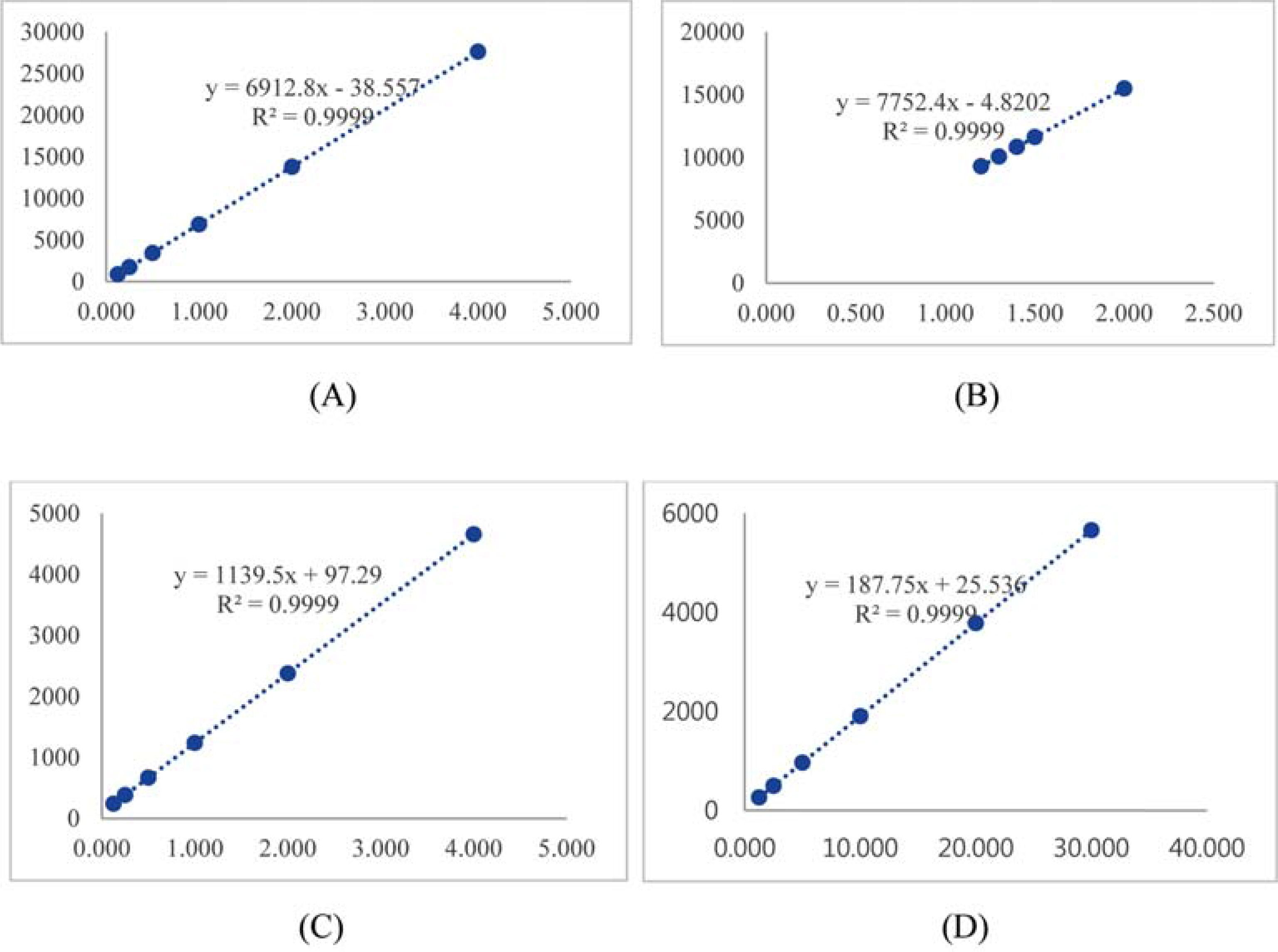
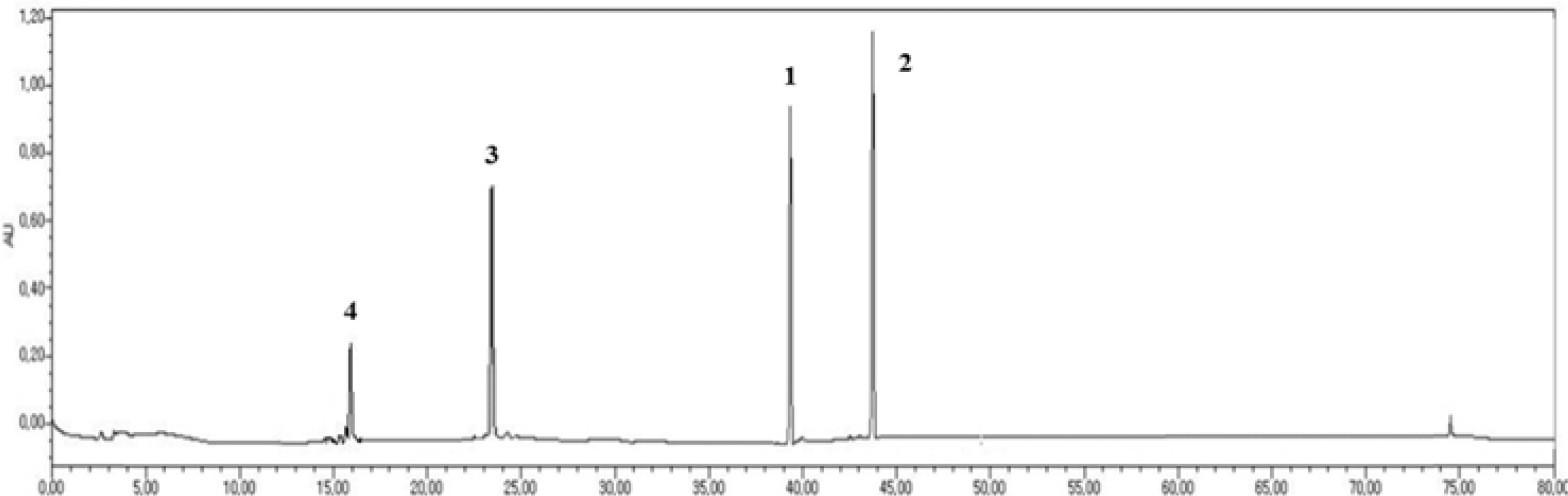
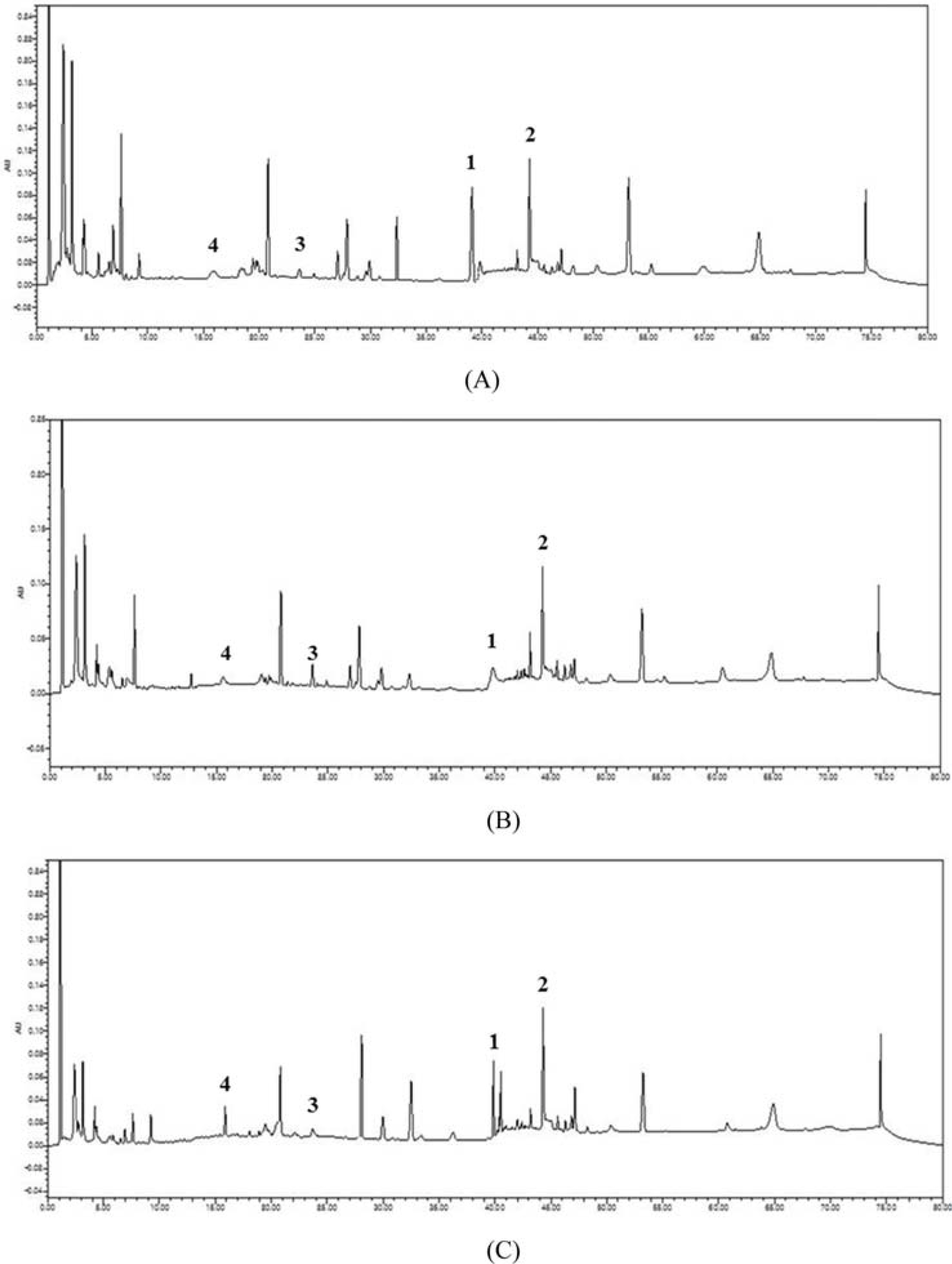
- Ginseng products available in different forms and preparations are reported to have varied bioactivities and chemical compositions. In our previous study, four new dammarane-type ginsenosides were isolated from Panax ginseng, which are ginsenoside Rg18 (1), 6-acetyl ginsenoside Rg3 (2), ginsenoside Rs11 (3), and ginsenoside Re7 (4). Accordingly, the goal of this study was to determine the distribution and content of these newly characterized ginsenosides in different ginseng products. The content of compounds 1 - 4 in different ginseng products was determined via HPLC-UV. The samples included ginseng roots from different ginseng species, roots harvested from different localities in Korea, and samples harvested at different cultivation ages and processed under different manufacturing methods. The four ginsenosides were present at varying concentrations in the different ginseng samples examined. The variations in their content could be attributed to species variation, and differences in cultivation conditions and manufacturing methods. The total concentration of compounds 1 - 4 were highest in ginseng obtained from Geumsan (185 µg/g), white-6 yr ginseng (150 µg/g), and P. quinquefolius (186 µg/g). The results of this study provide a basis for the optimization of cultivation conditions and manufacturing methods to maximize the yield of the four new ginsenosides in ginseng.
Keyword
MeSH Terms
Figure
Reference
-
(1). Li T.S.C.Hort. Technol. 1995; 5:27–34.(2). Coon J.T., Ernst E.Drug Safety. 2002; 25:323–344.(3). Attele A., Zhou P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P., Polonsky K., Yuan C.S.Diabetes. 2002; 51:1851–1858.(4). Shibata S. J.Korean Med. Sci. 2001; 16:S28–37.(4). Shibata S. J.Korean Med. Sci. 2001; 16:S28–37.(5). Siddiqi M.H., Siddiqi M.Z., Ahn S., Kang S., Kim Y.J., Sathishkumar N., Yang D.U., Yang D.C.J. Ginseng Res. 2013; 37:261–268.(6). Kitts D.D., Wijewickreme A.N., Hu C.Mol. Cell Biochem. 2000; 203:1–10.
Article(7). Karu N., Reifen R., Kerem Z. J.Agric. Food Chem. 2007; 55:2824–2828.(8). Yue P.Y.K., Mak N.K., Cheng Y.K., Leung K.W., Ng T.B., Fan D.T.P., Yeung H.W., Wong R.N.S.Chin. Med. 2007; 2:1–21.(9). Jang H.E., Jung H.J., Mok H.J.Appl. Biol. Chem. 2017; 60:321–326.(10). Proctor J.T., Bailey W.Hort. Rev. 1987; 9:187–236.(11). Baeg I.H., So S.H. J.Ginseng Res. 2013; 37:1–7.(12). Wang W., Zhao Y., Rayburn E., Hill D., Wang H., Zhang R.Cancer Chemother. Pharmacol. 2007; 59:589–601.(13). Wang J., Li S., Fan Y., Chen Y., Liu D., Cheng H., Gao X., Zhou Y. J.Ethnopharmacol. 2010; 130:421–423.(14). Matsunaga H., Mitsuo K., Yamamoto H., Fujito H., Mori M., Takata K.Chem. Pharm. Bull. 1990; 38:3480–3482.(15). Joo K.M., Park C.H., Jeong H.J., Lee S.J., Chang I.S. J.Chromatography B. 2008; 865:159–166.(16). Christensen L.Adv. Food. Nutr. Res. 2008; 55:1–99.(17). Lee D.G., Lee A.H., Kim K.T., Cho E.J., Lee S.Chem Pharm Bull. 2015; 63:927–934.(18). Lee C.R., Whang W.K., Shin C.G., Lee H.S., Han S.T., Im B.O., Ko S.K. Kor. J.Food Sci. Technol. 2004; 36:847–850.(19). Lee S.A., Liuting H.K., Im B.O., Cho S.H., Whang W.K., Ko S.K. Kor. J.Pharmacogn. 2010; 41:319–322.(20). Ahn S.I., Kim S.K., Yang B.W., Lee E.S., Kang C.S., Hahm Y.T. Kor. J.Hort. Sci. Tech. 2016; 34:790–798.(21). Dong H.D., Cho C.W., Kim Y.C., Kim E.Y., Rhee Y.K., Rho J.H., Choi S.H. J.Ginseng Res. 2012; 36:314–321.(22). Lim C.Y., Moon J.M., Kim B.Y., Lim S.H., Lee G.S., Yu H.S., Cho S.I. J.Ginseng Res. 2015; 39:38–45.(23). Cui J.F.Eur. J. Pharm. Sci. 1995; 3:77–85.(24). Keum Y.S., Park K.Y., Lee J.M., Chun K.S., Park J.H., Lee S.K., Kwon H.J., Surh Y.J.Cancer Lett. 2000; 150:41–48.(25). Kim D.H. J.Ginseng Res. 2012; 36:1–15.(26). Lee M.J., Choi J.S., Woo S.W., Lee K.S., Lee Z.W., Hwang G.S., Lee S.H., Kamal A.H.M., Jung Y.A., Seung N.S., Woo S.H.Process Biochem. 2011; 46:258–264.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Dammarane-type Triterpenoid Saponins from the Root of Panax ginseng
- Inhibition of TNF-alpha-Mediated NF-kappaB Transcriptional Activity by Dammarane-Type Ginsenosides from Steamed Flower Buds of Panax ginseng in HepG2 and SK-Hep1 Cells
- Brief Introduction of Panax ginseng C.A. Meyer
- Bioconversion of Ginsenosides from Red Ginseng Extract Using Candida allociferrii JNO301 Isolated from Meju
- Lipids in Ginseng (Panax ginseng) and Their Analysis