Korean J Physiol Pharmacol.
2018 Nov;22(6):637-647. 10.4196/kjpp.2018.22.6.637.
The treatment effect of novel hGHRH homodimer to male infertility hamster
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou 510006, China. songstang@hotmail.com
- 2Clinical Laboratories, Guangdong Provincial Corps Hospital of Chinese People's Armed Police Forces, Guangzhou Medical University, Guangzhou 510507, China.
- 3Department of Chemical & Biological Engineering, School of Engineering, University of Wisconsin-Madison, Madison 53706, USA.
- 4Department of Obstetrics & Gynecology, Guangdong Provincial Corps Hospital of Chinese People's Armed Police Forces, Guangzhou Medical University, Guangzhou 510507, China.
- 5Guangdong Provincial Key Laboratory of Pharmaceutical Bioactive Substances, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou 510006, China.
- 6Department of Endocrinology, Wuwei City Hospital, Wuwei 733000, China.
- KMID: 2430086
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.637
Abstract
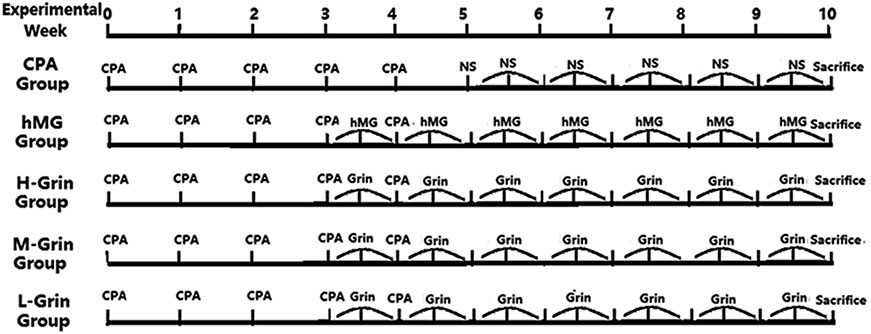
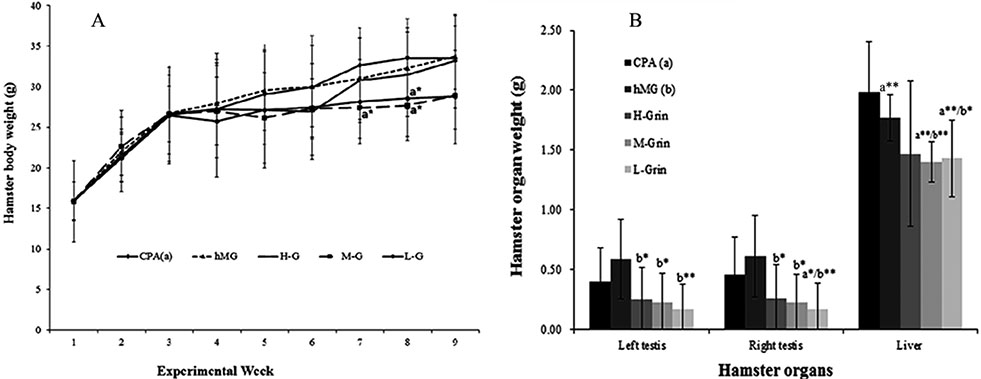
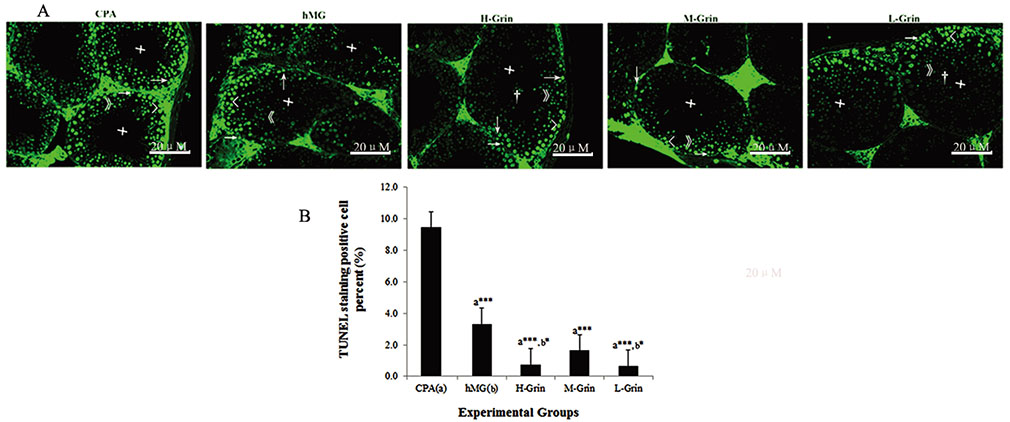
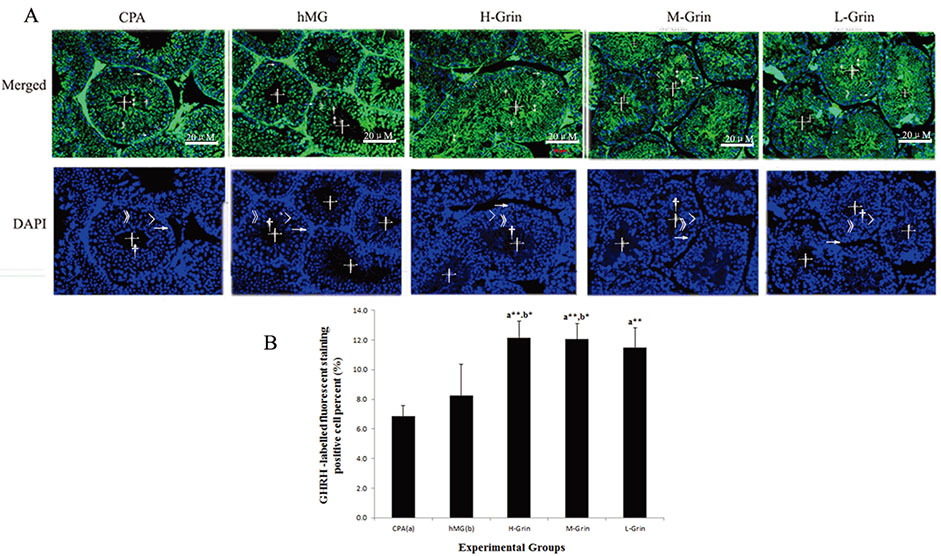
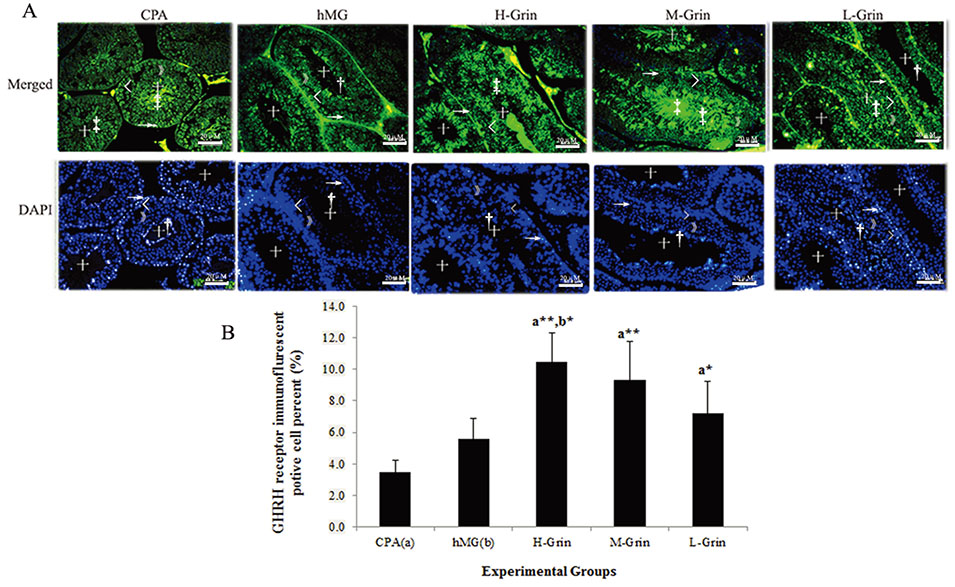
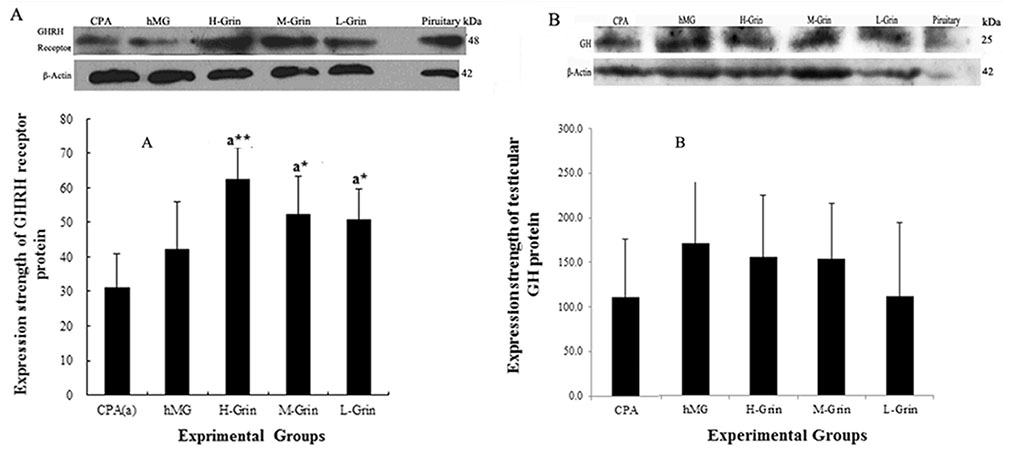
- Extra-hypothalamic growth hormone-releasing hormone (GHRH) plays an important role in reproduction. To study the treatment effect of Grin (a novel hGHRH homodimer), the infertility models of 85 male Chinese hamsters were established by intraperitoneally injecting 20 mg/kg of cyclophosphamide once in a week for 5 weeks and the treatment with Grin or human menopausal gonadotropin (hMG) as positive control was evaluated by performing a 3-week mating experiment. 2-8 mg/kg of Grin and 200 U/kg of hMG showed similar effect and different pathological characteristics. Compared to the single cyclophosphamide group (0%), the pregnancy rates (H-, M-, L-Grin 26.7, 30.8, 31.3%, and hMG 31.3%) showed significant difference, but there was no difference between the hMG and Grin groups. The single cyclophosphamide group presented loose tubules with pathologic vacuoles and significant TUNEL positive cells. Grin induced less weight of body or testis, compactly aligned tubules with little intra-lumens, whereas hMG caused more weight of body or testis, enlarging tubules with annular clearance. Grin presented a dose-dependent manner or cell differentiation-dependentincrease in testicular GHRH receptor, and did not impact the levels of blood and testicular GH, testosterone. Grin promotes fertility by proliferating and differentiating primitive cells through up-regulating testicular GHRH receptor without triggering GH secretion, which might solve the etiology of oligoasthenozoospermia.
MeSH Terms
Figure
Reference
-
1. Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998; 13:Suppl 1. 33–44.
Article2. Jung JH, Seo JT. Empirical medical therapy in idiopathic male infertility: promise or panacea? Clin Exp Reprod Med. 2014; 41:108–114.
Article3. Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES Jr. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012; 187:973–978.
Article4. Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011; (1):CD007411.
Article5. Hull KL, Harvey S. Growth hormone: a reproductive endocrine-paracrine regulator? Rev Reprod. 2000; 5:175–182.
Article6. Chubb C. Sexual behavior and fertility of little mice. Biol Reprod. 1987; 37:564–569.7. Bartke A, Chandrashekar V, Turyn D, Steger RW, Debeljuk L, Winters TA, Mattison JA, Danilovich NA, Croson W, Wernsing DR, Kopchick JJ. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice. Proc Soc Exp Biol Med. 1999; 222:113–123.8. Bartke A, Naar EM, Johnson L, May MR, Cecim M, Yun JS, Wagner TE. Effects of expression of human or bovine growth hormone genes on sperm production and male reproductive performance in four lines of transgenic mice. J Reprod Fertil. 1992; 95:109–118.
Article9. Champion ZJ, Vickers MH, Gravance CG, Breier BH, Casey PJ. Growth hormone or insulin-like growth factor-I extends longevity of equine spermatozoa in vitro. Theriogenology. 2002; 57:1793–1800.
Article10. Debeljuk L, Steger RW, Wright JC, Mattison J, Bartke A. Effects of overexpression of growth hormone-releasing hormone on the hypothalamo-pituitary-gonadal function in the mouse. Endocrine. 1999; 11:171–179.
Article11. Berry SA, Srivastava CH, Rubin LR, Phipps WR, Pescovitz OH. Growth hormone-releasing hormone-like messenger ribonucleic acid and immunoreactive peptide are present in human testis and placenta. J Clin Endocrinol Metab. 1992; 75:281–284.
Article12. Martínez-Moreno CG, López-Marín LM, Carranza M, Giterman D, Harvey S, Arámburo C, Luna M. Growth hormone (GH) and GH-releasing hormone (GHRH): Co-localization and action in the chicken testis. Gen Comp Endocrinol. 2014; 199:38–45.
Article13. Luna M, Martínez-Moreno CG, Ahumada-Solórzano MS, Harvey S, Carranza M, Arámburo C. Extrapituitary growth hormone in the chicken reproductive system. Gen Comp Endocrinol. 2014; 203:60–68.
Article14. Zhou D, You J, Li QY, Li HZ, Wu WF, Zhang XD, Zhang JH, Tang SS, Wang YK, Liu T. Synthesis and biological evaluation of novel structure-related hGHRH agonistic analogs. Growth Factors. 2015; 33:160–168.
Article15. Tripathi DN, Jena GB. Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology. 2008; 248:96–103.
Article16. Tang SS, Zhang JH, Liu HX, Li HZ. PC2/CPE-mediated pro-protein processing in tumor cells and its differentiated cells or tissues. Mol Cell Endocrinol. 2009; 303:43–49.
Article17. Lee LT, Siu FK, Tam JK, Lau IT, Wong AO, Lin MC, Vaudry H, Chow BK. Discovery of growth hormone-releasing hormones and receptors in nonmammalian vertebrates. Proc Natl Acad Sci U S A. 2007; 104:2133–2138.
Article18. Jain S, Desai N, Bhangoo A. Pathophysiology of GHRH-growth hormone-IGF1 axis. Rev Endocr Metab Disord. 2013; 14:113–118.19. Spiliotis BE. Growth hormone insufficiency and its impact on ovarian function. Ann N Y Acad Sci. 2003; 997:77–84.
Article20. Bartke A, Cecim M, Tang K, Steger RW, Chandrashekar V, Turyn D. Neuroendocrine and reproductive consequences of overexpression of growth hormone in transgenic mice. Proc Soc Exp Biol Med. 1994; 206:345–359.
Article21. Magon N, Singh S, Saxena A, Sahay R. Growth hormone in male infertility. Indian J Endocrinol Metab. 2011; 15:Suppl 3. S248–S249.
Article22. Giagulli VA. Absence of effect of recombinant growth hormone to classic gonadotropin treatment on spermatogenesis of patients with severe hypogonadotropic hypogonadism. Arch Androl. 1999; 43:47–53.
Article23. Chen T, Cheng HJ. Clinical efficacy and safety of recombinant human-growth hormone in the treatment of idiopathic asthenospermia. Zhonghua Nan Ke Xue. 2007; 13:233–236.24. Childs GV. Development of gonadotropes may involve cyclic transdifferentiation of growth hormone cells. Arch Physiol Biochem. 2002; 110:42–49.
Article25. Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res. 2000; 55:237–266.26. Chen C, Vincent JD, Clarke IJ. Ion channels and the signal transduction pathways in the regulation of growth hormone secretion. Trends Endocrinol Metab. 1994; 5:227–233.
Article27. Miller TL, Godfrey PA, Dealmeida VI, Mayo KE. The rat growth hormone-releasing hormone receptor gene: structure, regulation and generation of receptor isoforms with different signaling properties. Endocrinology. 1999; 140:4152–4165.28. Ramírez JL, Castaño JP, Torronteras R, Martínez-Fuentes AJ, Frawley LS, García-Navarro S, Gracia-Navarro F. Growth hormone (GH)-releasing factor differentially activates cyclic adenosine 3′, 5′-monophosphate- and inositol phosphate-dependent pathways to stimulate GH release in two porcine somatotrope subpopulations. Endocrinology. 1999; 140:1752–1759.29. Pombo CM, Zalvide J, Gaylinn BD, Diéguez C. Growth hormone-releasing hormone stimulates mitogen-activated protein kinase. Endocrinology. 2000; 141:2113–2119.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Establishment of Sperm Penetration Assay Using Cryopreserved Hamster Oocyte
- Erratum to: The treatment effect of novel hGHRH homodimer to male infertility hamster
- Genetic Causes in Male Infertility and Current Studies on Infertility Genes
- A case of prolactinoma with chief complaint of male infertility
- Treatment of Male Infertility