Ann Lab Med.
2018 Nov;38(6):585-590. 10.3343/alm.2018.38.6.585.
Weak D Testing is not Required for D− Patients With C−E− Phenotype
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. duck.cho@skku.edu
- 2Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Laboratory Medicine Gachon University Gil Medical Center, Incheon, Korea.
- 4Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University, Seoul, Korea.
- KMID: 2429125
- DOI: http://doi.org/10.3343/alm.2018.38.6.585
Abstract
- BACKGROUND
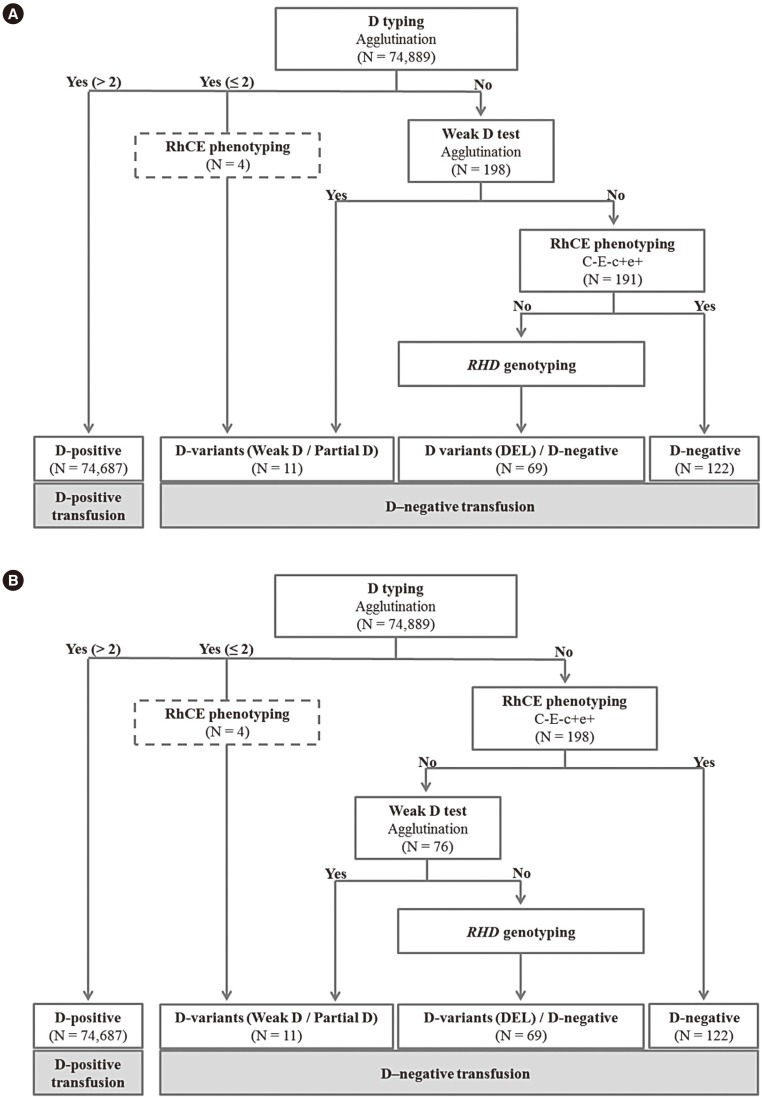
Although testing to detect weak D antigens using the antihuman globulin reagent is not required for D− patients in many countries, it is routinely performed in Korea. However, weak D testing can be omitted in D− patients with a C−E− phenotype as this indicates complete deletion of the RHD gene, except in rare cases. We designed a new algorithm for weak D testing, which consisted of RhCE phenotyping followed by weak D testing in C+ or E+ samples, and compared it with the current algorithm with respect to time and cost-effectiveness.
METHODS
In this retrospective study, 74,889 test results from January to July 2017 in a tertiary hospital in Korea were analyzed. Agreement between the current and proposed algorithms was evaluated, and total number of tests, time required for testing, and test costs were compared. With both algorithms, RHD genotyping was conducted for samples that were C+ or E+ and negative for weak D testing.
RESULTS
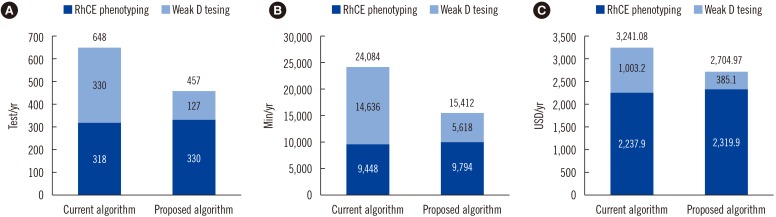
The algorithms showed perfect agreement (agreement=100%; κ=1.00). By applying the proposed algorithm, 29.56% (115/389 tests/yr) of tests could be omitted, time required for testing could be reduced by 36% (8,672/24,084 min/yr), and the test cost could be reduced by 16.53% (536.11/3,241.08 USD/yr).
CONCLUSIONS
Our algorithm omitting weak D testing in D− patients with C−E− phenotype may be a cost-effective testing strategy in Korea.
Keyword
Figure
Reference
-
1. Sandler SG, Flegel WA, Westhoff CM, Denomme GA, Delaney M, Keller MA, et al. It's time to phase in RHD genotyping for patients with a serologic weak D phenotype. College of American Pathologists Transfusion Medicine Resource Committee Work Group. Transfusion. 2015; 55:680–689. PMID: 25438646.2. Daniels G. Variants of RhD--current testing and clinical consequences. Br J Haematol. 2013; 161:461–470. PMID: 23432139.3. Jenkins CM, Johnson ST, Bellissimo DB, Gottschall JL. Incidence of weak D in blood donors typed as D positive by the Olympus PK 7200. Immunohematology. 2005; 21:152–154. PMID: 16472016.4. Sandler SG, Roseff SD, Domen RE, Shaz B, Gottschall JL. Policies and procedures related to testing for weak D phenotypes and administration of Rh immune globulin: results and recommendations related to supplemental questions in the Comprehensive Transfusion Medicine survey of the College of American Pathologists. Arch Pathol Lab Med. 2014; 138:620–625. PMID: 24786120.5. AABB. Standards for Blood Banks and Transfusion services. 18th ed. American Association of Blood Banks;2016. p. 327–328.6. Han KS, Park KU, Song EY. Transfusion medicine. 4th ed. Seoul: Korea Medical Book Publishing Company;2014. p. 367–370.7. Japan Society of Transfusion Medicine and Cell Therapy, Transfusion examination technology training course committee Ver.1.3.1. Inspection manual for blood transfusion. Tokyo: Japan Society of Transfusion Medicine and Cell Therapy, Transfusion examination technology training course committee;2017. p. 3–5.8. Hors collection. Transfusion medicine: the French model. Montrouge: John Libbey Eurotext;2013. p. 61–82.9. Kim JY, Kim SY, Kim CA, Yon GS, Park SS. Molecular characterization of D- Korean persons: development of a diagnostic strategy. Transfusion. 2005; 45:345–352. PMID: 15752151.10. Laboratory Medicine Foundation. 2018 Checklist for Excellent Laboratory Certification_Transfusion Medicine. Updated on Dec 2017. http://lmf.or.kr/sub/catalog.11. Shao CP. Transfusion of RhD-positive blood in “Asia type” DEL recipients. N Engl J Med. 2010; 362:472–473. PMID: 20130261.12. Seo MH, Won EJ, Hong YJ, Chun S, Kwon JR, Choi YS, et al. An effective diagnostic strategy for accurate detection of RhD variants including Asian DEL type in apparently RhD-negative blood donors in Korea. Vox Sang. 2016; 111:425–430. PMID: 27864976.13. Lim YA, Cho HS, Choi YS, Jang CH, Lee MN, Kwon JR, et al. Report on external proficiency testing for the ABO and D blood group typing tests in blood centers (2015). Korean J Blood Transfus. 2016; 27:68–78.14. Korean Medical Association. Health insurance review and assessment service of Korea. Seoul: Kyungsung Media;2017. p. 110.15. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.16. Sandler SG, Chen LN, Flegel WA. Serological weak D phenotypes: a review and guidance for interpreting the RhD blood type using the RHD genotype. Br J Haematol. 2017; 179:10–19. PMID: 28508413.17. Pham BN, Roussel M, Peyrard T, Beolet M, Jan-Lasserre V, Gien D, et al. Anti-D investigations in individuals expressing weak D Type 1 or weak D Type 2: allo- or autoantibodies? Transfusion. 2011; 51:2679–2685. PMID: 21658048.18. Flegel WA, von Zabern I, Wagner FF. Six years' experience performing RHD genotyping to confirm D- red blood cell units in Germany for preventing anti-D immunizations. Transfusion. 2009; 49:465–471. PMID: 19243542.19. Ogasawara K, Suzuki Y, Sasaki K, Osabe T, Isa K, Tsuneyama H, et al. Molecular basis for D- Japanese: identification of novel DEL and D- alleles. Vox Sang. 2015; 109:359–365. PMID: 25953588.20. Wang M, Wang BL, Xu W, Fan DD, Peng ML, Pan J, et al. Anti-D alloimmunisation in pregnant women with DEL phenotype in China. Transfus Med. 2015; 25:163–169. PMID: 26033335.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Weak D type 33 Found in a Patient with a Weak D Phenotype: The First Case in Korea
- Weak D Type 102 Found in a Family Study: The First Case in Korea
- Multiple Group Testing Procedures for Analysis of High-Dimensional Genomic Data
- An Algorithm to Work-up ABO Subgroups Presenting as Weak B in a Real-world Laboratory: A Case with a Weak B Phenotype Harboring B101/O04-variant Alleles
- Relation between Ischemia on Exercise Testing and on Holter Monitoring