Ann Lab Med.
2018 Nov;38(6):555-562. 10.3343/alm.2018.38.6.555.
Detection of mcr-1 Plasmids in Enterobacteriaceae Isolates From Human Specimens: Comparison With Those in Escherichia coli Isolates From Livestock in Korea
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. kscpjsh@yuhs.ac
- 2Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3National Institute of Health, Centers for Disease Control and Prevention, Cheongju, Korea.
- KMID: 2429121
- DOI: http://doi.org/10.3343/alm.2018.38.6.555
Abstract
- BACKGROUND
The emerging mobile colistin resistance gene, mcr-1, is an ongoing worldwide concern and an evaluation of clinical isolates harboring this gene is required in Korea. We investigated mcr-1-possessing Enterobacteriaceae among Enterobacteriaceae strains isolated in Korea, and compared the genetic details of the plasmids with those in Escherichia coli isolates from livestock.
METHODS
Among 9,396 Enterobacteriaceae clinical isolates collected between 2010 and 2015, 1,347 (14.3%) strains were resistant to colistin and those were screened for mcr-1 by PCR. Colistin minimum inhibitory concentrations (MICs) were determined by microdilution, and conjugal transfer of the mcr-1-harboring plasmids was assessed by direct mating. Whole genomes of three mcr-1-positive Enterobacteriaceae clinical isolates and 11 livestock-origin mcr-1-positive E. coli isolates were sequenced.
RESULTS
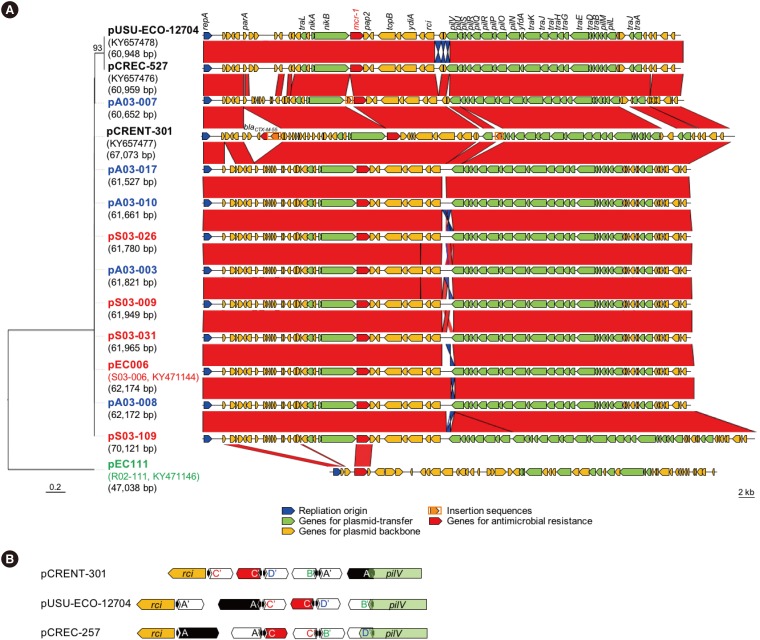
Two E. coli and one Enterobacter aerogenes clinical isolates carried carried IncI2 plasmids harboring mcr-1, which conferred colistin resistance (E. coli MIC, 4 mg/L; E. aerogenes MIC, 32 mg/L). The strains possessed the complete conjugal machinery except for E. aerogenes harboring a truncated prepilin peptidase. The E. coli plasmid transferred more efficiently to E. coli than to Klebsiella pneumoniae or Enterobacter cloacae recipients. Among the three bacterial hosts, the colistin MIC was the highest for E. coli owing to the higher mcr-1-plasmid copy number and mcr-1 expression levels. Ten mcr-1-positive chicken-origin E. coli strains also possessed mcr-1-harboring IncI2 plasmids closely related to that in the clinical E. aerogenes isolate, and the remaining one porcine-origin E. coli possessed an mcr-1-harboring IncX4 plasmid.
CONCLUSIONS
mcr-1-harboring IncI2 plasmids were identified in clinical Enterobacteriaceae isolates. These plasmids were closely associated with those in chicken-origin E. coli strains in Korea, supporting the concept of mcr-1 dissemination between humans and livestock.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
MCR1 and KPC2 Co-producing Klebsiella pneumoniae Bacteremia: First Case in Korea
Ji Young Park, Sang Taek Heo, Ki Tae Kwon, Do Young Song, Kwang Jun Lee, Ji Ae Choi
Infect Chemother. 2019;51(4):399-404. doi: 10.3947/ic.2019.51.4.399.
Reference
-
1. Giamarellou H, Poulakou G. Multidrug-resistant gram-negative infections: what are the treatment options? Drugs. 2009; 69:1879–1901. PMID: 19747006.2. Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016; 16:161–168. PMID: 26603172.3. Skov RL, Monnet DL. Plasmid-mediated colistin resistance (mcr-1 gene): three months later, the story unfolds. Euro Surveill. 2016; 21:30155. PMID: 26967914.4. Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S, et al. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother. 2016; 60:5612–5615. PMID: 27401575.5. Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016; 21:30280.6. Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio. 2017; 8:e00543-17. PMID: 28655818.7. Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, et al. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017; 22:30589. PMID: 28797329.8. Shen Z, Wang Y, Shen Y, Shen J, Wu C. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect Dis. 2016; 16:293. PMID: 26973308.9. von Wintersdorff CJ, Wolffs PF, van Niekerk JM, Beuken E, van Alphen LB, Stobberingh EE, et al. Detection of the plasmid-mediated colistin-resistance gene mcr-1 in faecal metagenomes of Dutch travellers. J Antimicrob Chemother. 2016; 71:3416–3419. PMID: 27559117.10. Robinson TP, Bu DP, Carrique-Mas J, Fèvre EM, Gilbert M, Grace D, et al. Antibiotic resistance is the quintessential One Health issue. Trans R Soc Trop Med Hyg. 2016; 110:377–380. PMID: 27475987.11. Gao R, Hu Y, Li Z, Sun J, Wang Q, Lin J, et al. Dissemination and mechanism for the MCR-1 colistin resistance. PLoS Pathog. 2016; 12:e1005957. PMID: 27893854.12. Falgenhauer L, Waezsada SE, Yao Y, Imirzalioglu C, Kasbohrer A, Roesler U, et al. Colistin resistance gene mcr-1 in extended-spectrum beta-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect Dis. 2016; 16:282–283. PMID: 26774242.13. Snesrud E, Ong AC, Corey B, Kwak YI, Clifford R, Gleeson T, et al. Analysis of Serial Isolates of mcr-1-positive Escherichia coli reveals a highly active ISApl1 transposon. Antimicrob Agents Chemother. 2017; 61:e00056-17. PMID: 28223389.14. Du H, Chen L, Tang YW, Kreiswirth BN. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis. 2016; 16:287–288. PMID: 26842776.15. Zhang XF, Doi Y, Huang X, Li HY, Zhong LL, Zeng KJ, et al. Possible transmission of mcr-1-harboring Escherichia coli between companion animals and human. Emerg Infect Dis. 2016; 22:1679–1681. PMID: 27191649.16. Yang RS, Feng Y, Lv XY, Duan JH, Chen J, Fang LX, et al. Emergence of NDM-5- and MCR-1-producing Escherichia coli clones ST648 and ST156 from a single muscovy duck (Cairina moschata). Antimicrob Agents Chemother. 2016; 60:6899–6902. PMID: 27550364.17. Teo JQ, Ong RT, Xia E, Koh TH, Khor CC, Lee SJ, et al. mcr-1 in multidrug-resistant blaKPC-2-producing clinical Enterobacteriaceae isolates in Singapore. Antimicrob Agents Chemother. 2016; 60:6435–6437. PMID: 27503652.18. Sun J, Li XP, Yang RS, Fang LX, Huo W, Li SM, et al. Complete nucleotide sequence of an IncI2 plasmid coharboring blaCTX-M-55 and mcr-1. Antimicrob Agents Chemother. 2016; 60:5014–5017. PMID: 27216063.19. Quan J, Li X, Chen Y, Jiang Y, Zhou Z, Zhang H, et al. Prevalence of mcr-1 in Escherichia coli and Klebsiella pneumoniae recovered from bloodstream infections in China: a multicentre longitudinal study. Lancet Infect Dis. 2017; 17:400–410. PMID: 28139430.20. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010; 363:2377–2379. PMID: 21158655.21. Lim SK, Kang HY, Lee K, Moon DC, Lee HS, Jung SC. First detection of the mcr-1 gene in Escherichia coli isolated from livestock between 2013 and 2015 in South Korea. Antimicrob Agents Chemother. 2016; 60:6991–6993. PMID: 27572390.22. The Joint CLSI-European Committee on Antimicrobial Susceptibility Testing (EUCAST) Polymyxin Breakpoints Working Group. Recommendations for MIC determination of colistin (polymyxin E). Wayne, PA: Clinical and Laboratory Standards Institute;2016. Updated on Mar 2016. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf.23. Hong JS, Yoon EJ, Lee H, Jeong SH, Lee K. Clonal Dissemination of Pseudomonas aeruginosa sequence type 235 isolates carrying blaIMP-6 and emergence of blaGES-24 and blaIMP-10 on novel genomic islands PAGI-15 and -16 in South Korea. Antimicrob Agents Chemother. 2016; 60:7216–7223. PMID: 27671068.24. Poirel L, Kieffer N, Brink A, Coetze J, Jayol A, Nordmann P. Genetic features of MCR-1-producing colistin-resistant Escherichia coli isolates in South Africa. Antimicrob Agents Chemother. 2016; 60:4394–4397. PMID: 27161623.25. Kim ES, Chong YP, Park SJ, Kim MN, Kim SH, Lee SO, et al. Detection and genetic features of MCR-1-producing plasmid in human Escherichia coli infection in South Korea. Diagn Microbiol Infect Dis. 2017; 89:158–160. PMID: 28780246.26. Fernandes MR, McCulloch JA, Vianello MA, Moura Q, Pérez-Chaparro PJ, Esposito F, et al. First report of the globally disseminated IncX4 plasmid carrying the mcr-1 gene in a colistin-resistant Escherichia coli Sequence Type 101 isolate from a human infection in Brazil. Antimicrob Agents Chemother. 2016; 60:6415–6417. PMID: 27503650.27. Elnahriry SS, Khalifa HO, Soliman AM, Ahmed AM, Hussein AM, Shimamoto T, et al. Emergence of plasmid-mediated colistin resistance gene mcr-1 in a clinical Escherichia coli isolate from Egypt. Antimicrob Agents Chemother. 2016; 60:3249–3250. PMID: 26953204.28. Bradley DE, Coetzee JN. The determination of two morphologically distinct types of pilus by plasmids of incompatibility group I2. J Gen Microbiol. 1982; 128:1923–1926. PMID: 6128374.29. Yoshida T, Kim SR, Komano T. Twelve pil genes are required for biogenesis of the R64 thin pilus. J Bacteriol. 1999; 181:2038–2043. PMID: 10094679.30. Akahane K, Sakai D, Furuya N, Komano T. Analysis of the pilU gene for the prepilin peptidase involved in the biogenesis of type IV pili encoded by plasmid R64. Mol Genet Genomics. 2005; 273:350–359. PMID: 15838638.31. Komano T, Kim SR, Yoshida T, Nisioka T. DNA rearrangement of the shufflon determines recipient specificity in liquid mating of IncI1 plasmid R64. J Mol Biol. 1994; 243:6–9. PMID: 7932741.32. Sun J, Fang LX, Wu Z, Deng H, Yang RS, Li XP, et al. Genetic analysis of the IncX4 plasmids: implications for a unique pattern in the mcr-1 acquisition. Sci Rep. 2017; 7:424. PMID: 28336940.33. Yoon EJ, Yang JW, Kim JO, Lee H, Lee KJ, Jeong SH. Carbapenemase-producing Enterobacteriaceae in South Korea: a report from the National Laboratory Surveillance System. Future Microbiol. 2018; 13:771–783. PMID: 29478336.34. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32:1792–1797. PMID: 15034147.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Colistin resistance and plasmidmediated mcr genes in Escherichia coli and Salmonella isolated from pigs, pig carcass and pork in Thailand, Lao PDR and Cambodia border provinces
- Antimicrobial Resistance and Integrons Found in Commensal Escherichia coli Isolates from Healthy Humans
- The characteristics of extended-spectrum beta-lactamases in Korean isolates of Enterobacteriaceae
- Comparisons of CTX-M-Producing Escherichia coli Isolates from Humans and Animals in South Korea
- Dissemination of Plasmid-mediated qnr, aac(6')-Ib-cr, and qepA Genes Among 16S rRNA Methylase Producing Enterobacteriaceae in Korea