J Pathol Transl Med.
2018 Nov;52(6):369-377. 10.4132/jptm.2018.09.19.
High Cytoplasmic CXCR4 Expression Predicts Prolonged Survival in Triple-Negative Breast Cancer Patients Treated with Adjuvant Chemotherapy
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. karlnash@naver.com
- 2Department of Pathology, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea.
- KMID: 2427519
- DOI: http://doi.org/10.4132/jptm.2018.09.19
Abstract
- BACKGROUND
Chemokine receptor CXC chemokine receptor type 4 (CXCR4) and its ligand CXC motif chemokine 12 (CXCL12; stromal cell-derived factor-1) are implicated in tumor growth, metastasis, and tumor cell-microenvironment interaction. A number of studies have reported that increased CXCR4 expression is associated with worse prognosis in triple-negative breast cancer (TNBC), but its prognostic significance has not been studied in TNBC patients treated with adjuvant chemotherapy.
METHODS
Two hundred eighty-three TNBC patients who received adjuvant chemotherapy were retrospectively analyzed. Tissue microarray was constructed from formalin-fixed, paraffin-embedded tumor tissue and immunohistochemistry for CXCR4 and CXCL12 was performed. Expression of each marker was compared with clinicopathologic characteristics and outcome.
RESULTS
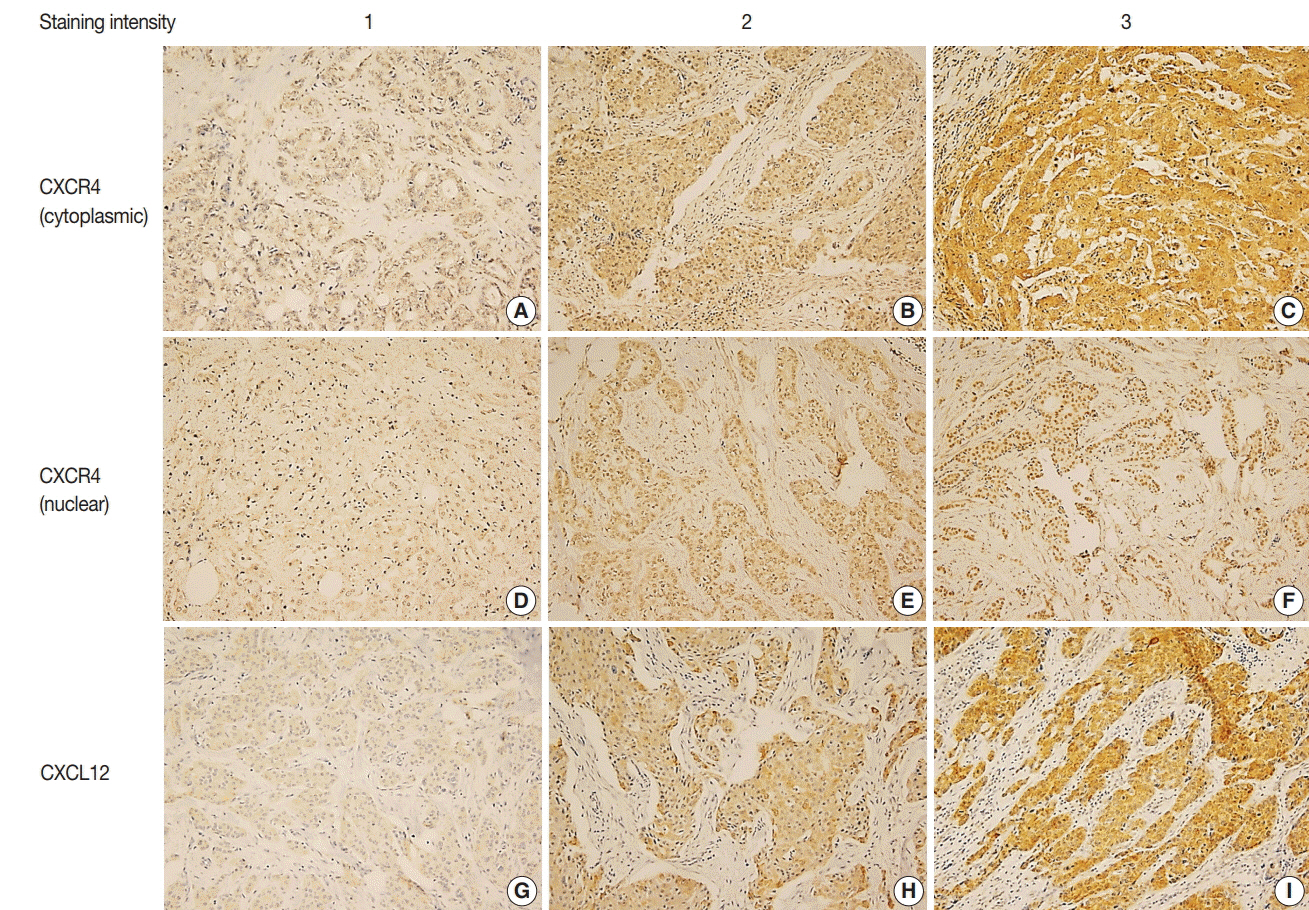
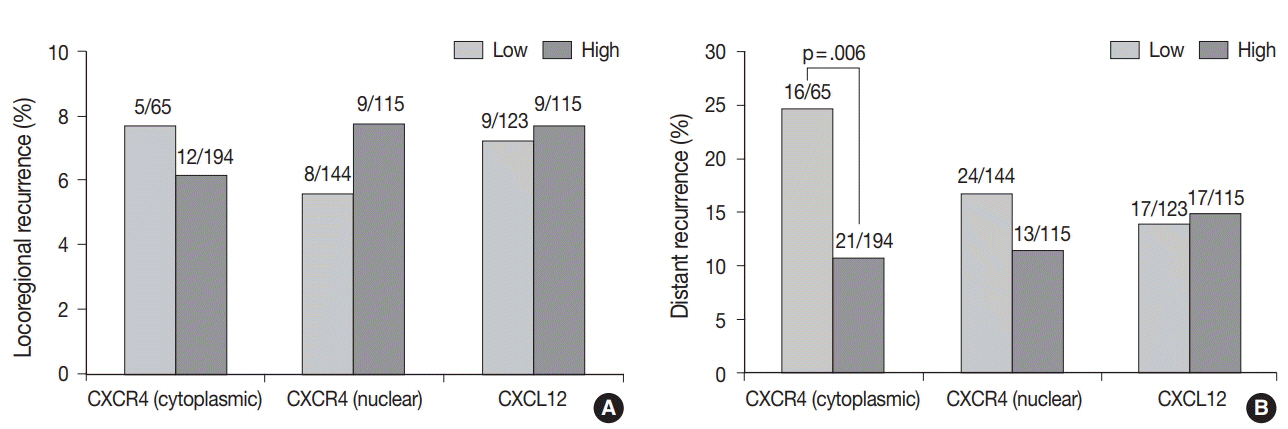
High cytoplasmic CXCR4 expression was associated with younger age (p = .008), higher histologic grade (p = .007) and lower pathologic stage (p = .045), while high CXCL12 expression was related to larger tumor size (p = .045), positive lymph node metastasis (p = .005), and higher pathologic stage (p = .017). The patients with high cytoplasmic CXCR4 experienced lower distant recurrence (p = .006) and better recurrence-free survival (RFS) (log-rank p = .020) after adjuvant chemotherapy. Cytoplasmic CXCR4 expression remained an independent factor of distant recurrence (p = .019) and RFS (p = .038) after multivariate analysis.
CONCLUSIONS
High cytoplasmic CXCR4 expression was associated with lower distant recurrence and better RFS in TNBC patients treated with adjuvant chemotherapy. This is the first study to correlate high CXCR4 expression to better TNBC prognosis, and the underlying mechanism needs to be elucidated in further studies.
MeSH Terms
Figure
Reference
-
1. Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012; 23 Suppl 6:vi7–12.
Article2. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016; 13:674–90.
Article3. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004; 4:540–50.
Article4. Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996; 382:635–8.
Article5. Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998; 392:565–8.
Article6. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998; 393:595–9.
Article7. Domanska UM, Kruizinga RC, Nagengast WB, et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013; 49:219–30.
Article8. Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005; 121:335–48.
Article9. Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001; 410:50–6.
Article10. Liu Y, Ji R, Li J, et al. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010; 29:16.
Article11. Zhang Z, Ni C, Chen W, et al. Expression of CXCR4 and breast cancer prognosis: a systematic review and meta-analysis. BMC Cancer. 2014; 14:49.
Article12. Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004; 64:8604–12.
Article13. Huang EH, Singh B, Cristofanilli M, et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J Surg Res. 2009; 155:231–6.
Article14. Chu QD, Panu L, Holm NT, Li BD, Johnson LW, Zhang S. High chemokine receptor CXCR4 level in triple negative breast cancer specimens predicts poor clinical outcome. J Surg Res. 2010; 159:689–95.
Article15. Yu S, Wang X, Liu G, Zhu X, Chen Y. High level of CXCR4 in triplenegative breast cancer specimens associated with a poor clinical outcome. Acta Med Okayama. 2013; 67:369–75.16. Chen HW, Du CW, Wei XL, Khoo US, Zhang GJ. Cytoplasmic CXCR4 high-expression exhibits distinct poor clinicopathological characteristics and predicts poor prognosis in triple-negative breast cancer. Curr Mol Med. 2013; 13:410–6.
Article17. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–95.
Article18. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013; 31:3997–4013.19. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–10.
Article20. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007; 25:2127–32.
Article21. Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016; 35:816–26.
Article22. Haribabu B, Richardson RM, Fisher I, et al. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997; 272:28726–31.23. Salvucci O, Bouchard A, Baccarelli A, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006; 97:275–83.
Article24. Hassan S, Ferrario C, Saragovi U, et al. The influence of tumor-host interactions in the stromal cell-derived factor-1/CXCR4 ligand/receptor axis in determining metastatic risk in breast cancer. Am J Pathol. 2009; 175:66–73.
Article25. Guembarovski AL, Guembarovski RL, Hirata BK, et al. CXCL12 chemokine and CXCR4 receptor: association with susceptibility and prognostic markers in triple negative breast cancer. Mol Biol Rep. 2018; Jun. 20. [Epub]. https://doi.org/10.1007/s11033-018-4215-7.
Article26. Kobayashi T, Tsuda H, Moriya T, et al. Expression pattern of stromal cell-derived factor-1 chemokine in invasive breast cancer is correlated with estrogen receptor status and patient prognosis. Breast Cancer Res Treat. 2010; 123:733–45.
Article27. Mirisola V, Zuccarino A, Bachmeier BE, et al. CXCL12/SDF1 expression by breast cancers is an independent prognostic marker of diseasefree and overall survival. Eur J Cancer. 2009; 45:2579–87.
Article28. Yan M, Jene N, Byrne D, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011; 13:R47.
Article29. Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005; 7:R402–10.
Article30. Samarendra H, Jones K, Petrinic T, et al. A meta-analysis of CXCL12 expression for cancer prognosis. Br J Cancer. 2017; 117:124–35.
Article31. Lefort S, Thuleau A, Kieffer Y, et al. CXCR4 inhibitors could benefit to HER2 but not to triple-negative breast cancer patients. Oncogene. 2017; 36:1211–22.
Article32. Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018; 25(Suppl 1):S142–S50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Survival Benefit of Different Chemotherapy Regimens in Patients with T1-2N0 Triple-Negative Breast Cancer
- The Prognostic Significance of Survivin Expression in Breast Cancer
- Association of CXCR4 Expression with Metastasis and Survival among Patients with Non-small Cell Lung Cancer
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Response to Paclitaxel in Node-positive Triple Negative Breast Cancer