J Korean Ophthalmol Soc.
2010 May;51(5):707-715.
Intravitreal Bevacizumab Treatment of Macular Edema in Central Retinal Vein Occlusion
- Affiliations
-
- 1Department of Ophthalmology, Pusan Paik Hospital, Inje University College of Medicine, Busan, Korea. maekbak@hanmail.net
Abstract
- PURPOSE
To report the effect of intravitreal injection of bevacizumab for the treatment of macular edema due to central retinal vein occlusion (CRVO).
METHODS
In a retrospective study, 18 consecutive patients (18 eyes) with macular edema from CRVO received intravitreal bevacizumab (1.25 mg). Ophthalmic examination included best corrected visual acuity (BCVA) and central macular thickness (CMT) at baseline and follow-up visits. Fluorescein angiography was performed during follow-up visits if necessary. Primary outcomes included a change in BCVA and CMT.
RESULTS
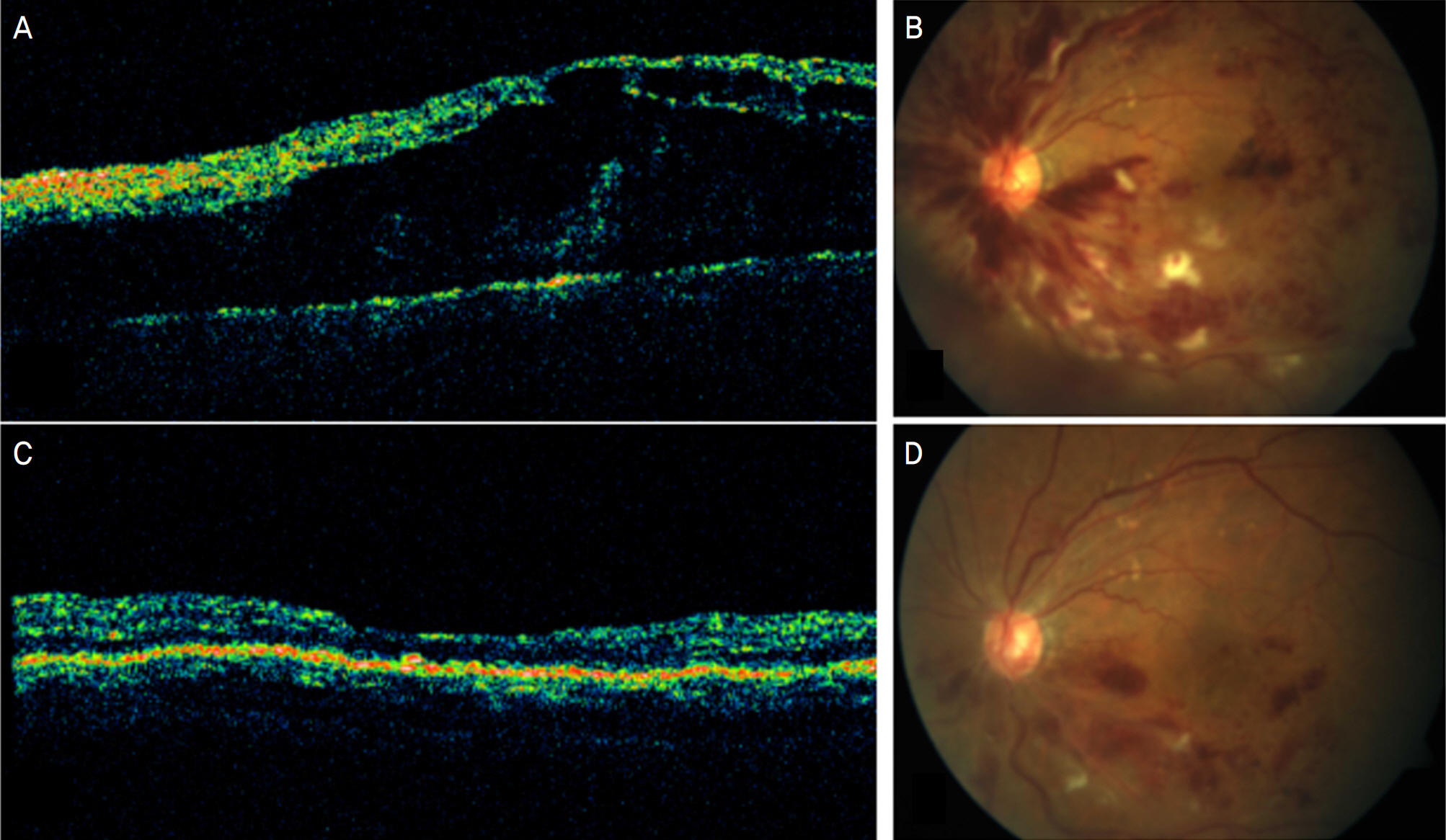
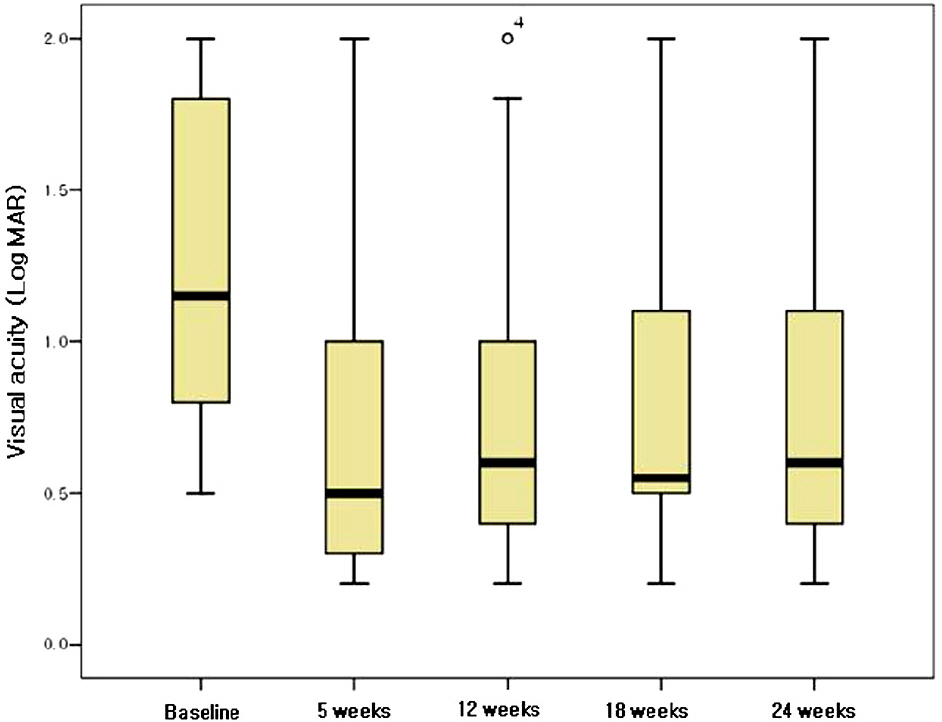
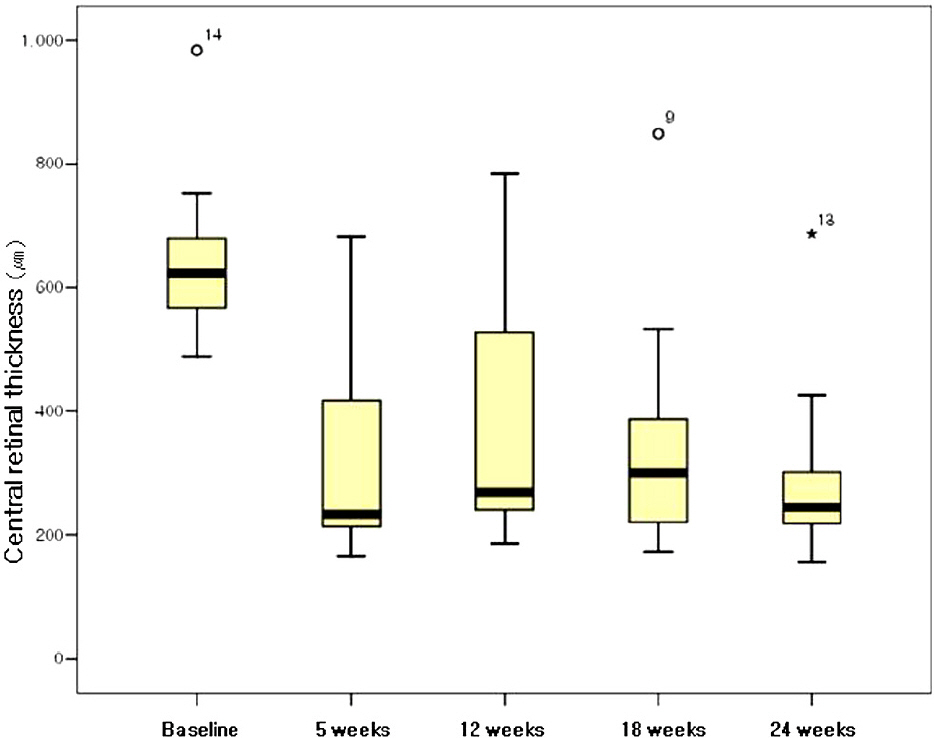
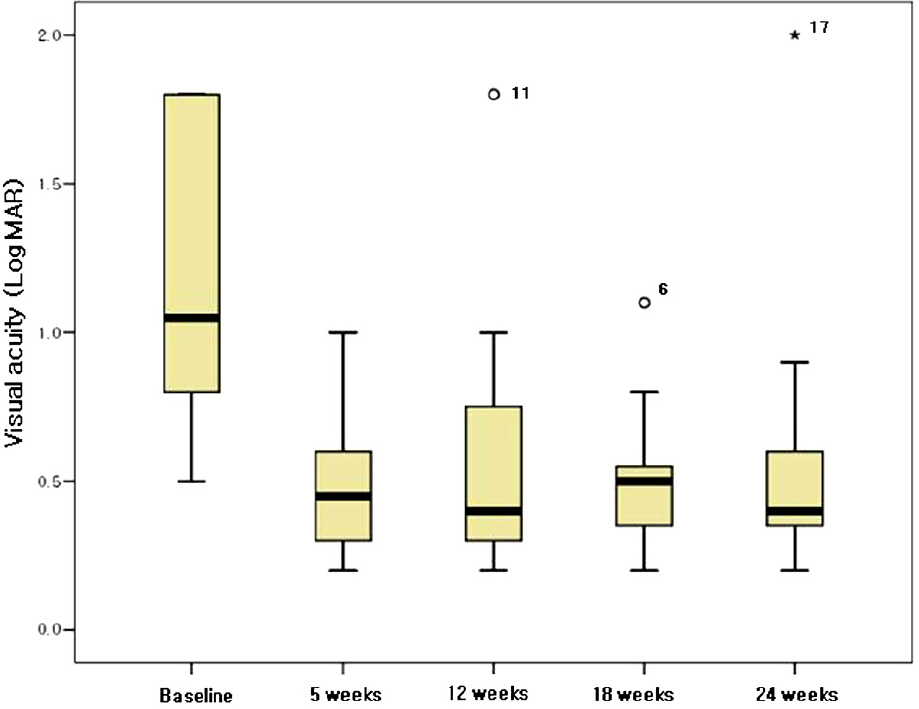
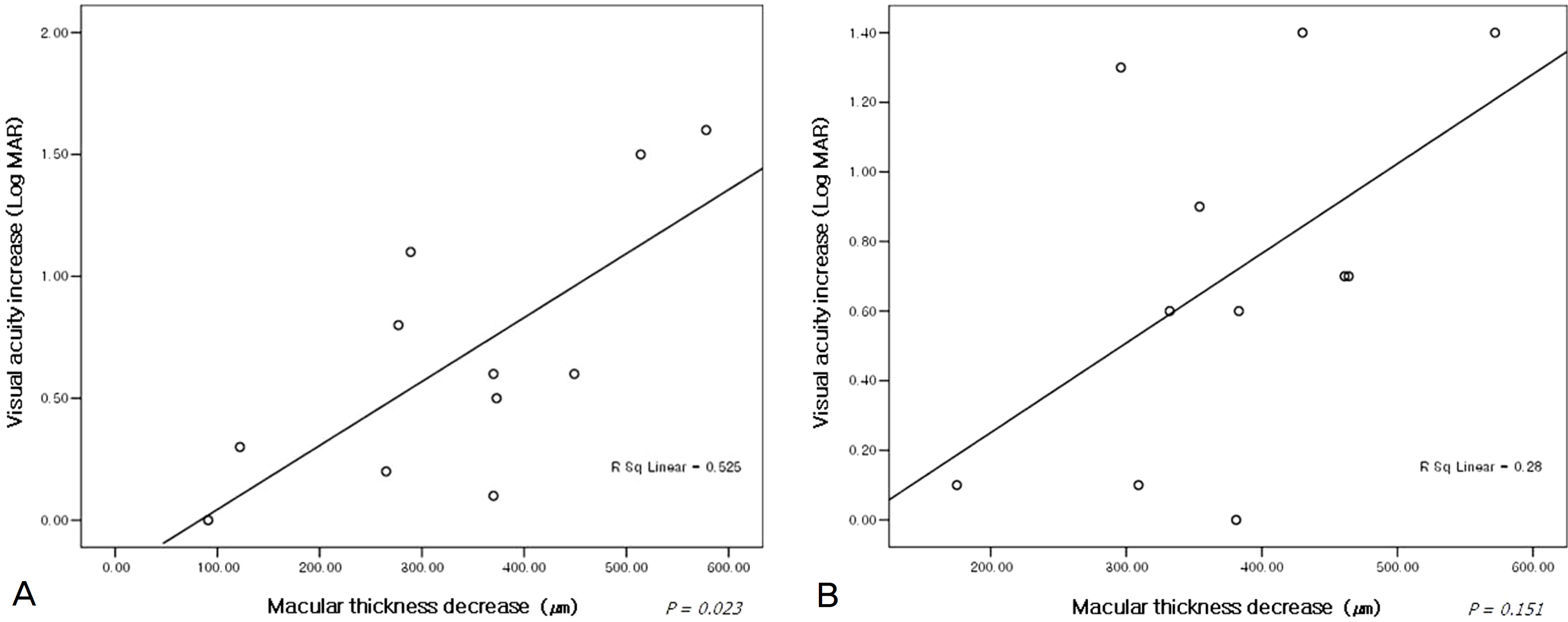
The mean duration from symptom detection to the first bevacizumab injection was 32.5 days. The patients received a mean of 2.17 injections of bevacizumab per eye. The mean baseline visual acuity (LogMAR) was 1.27 and increased to a mean of 0.75 at 5 weeks, and 0.81 at 24 weeks. The mean central macular thickness at baseline was 640.5 micrometer and decreased to a mean of 295.6 micrometer at 5 weeks and 284.7 micrometer at 24 weeks (p<0.05). In the ischemic CRVO group, no significant changes in visual acuity were found after 24 weeks. The increase in visual acuity did not correlate significantly with the decrease in CMT after 24 weeks (p=0.205). The result from the non-ischemic group was similar to the preceding result (p=0.151).
CONCLUSIONS
Intravitreal bevacizumab resulted in a significant decrease in CMT in patients with CRVO after a 6-month follow-up. The visual acuity in patients with non-ischemic CRVO improved, but there was no significant improvement in the ischemic CRVO group.
MeSH Terms
Figure
Reference
-
References
1. Central vein occlusion study of photocoagulation therapy. Baseline findings. Central Vein Occlusion Study Group. Online J Curr Clin Trials. 1993. Doc No 95:[6021 words;81 paragraphs].2. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997; 115:486–91.3. Central vein occlusion study of photocoagulation. Manual of operations. Central Vein Occlusion Study Group. Online J Curr Clin Trials. 1993. Doc No 92:[32,228 words;678 paragraphs].4. Green WR, Chan CC, Hutchins GM, Terry JM. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc. 1981; 79:371–422.5. Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992; 131:710–4.
Article6. Cohen T, Nahari D, Cerem LW, et al. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996; 271:736–41.
Article7. Bresnick GH. Diabetic maculopathy. A critical review high-lighting diffuse macular edema. Ophthalmology. 1983; 90:1301–17.8. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.
Article9. Brooks HL Jr, Caballero S Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema abdominal and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004; 122:1801–7.10. Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005; 140:256–61.
Article11. Funatsu H, Yamashita H, Nakamura S, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006; 113:294–301.
Article12. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995; 102:1425–33.13. Jonas JB, Akkoyun I, Kamppeter B, et al. Intravitreal triamcinolone acetonide for treatment of central retinal vein occlusion. Eur J Ophthalmol. 2005; 15:751–8.
Article14. Jonas JB. Intravitreal triamcinolone acetonide for treatment of abdominal oedematous and neovascular diseases. Acta Ophthalmol Scand. 2005; 83:645–63.15. Gregori NZ, Rosenfeld PJ, Puliafito CA, et al. One-year safety and efficacy of intravitreal triamcinolone acetonide for the abdominal of macular edema secondary to central retinal vein occlusion. Retina. 2006; 26:889–95.16. Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal abdominal acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002; 86:247–8.17. Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the abdominal of cystoid macular edema. Am J Ophthalmol. 2005; 140:695–702.18. Gelston CD, Olson JL, Mandava N. Macular oedema in central retinal vein occlusion treated with intravitreal triamcinolone. Acta Ophthalmol Scand. 2006; 84:314–8.
Article19. Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as abdominal for macular edema from central retinal vein occlusion. Arch Ophthalmol. 2002; 120:1217–9.20. Pe'er J, Shweiki D, Itin A, et al. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995; 72:638–45.21. Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous abdominals of vascular endothelial growth factor produce retinal abdominal and microangiopathy in an adult primate. Ophthalmology. 1996; 103:1820–8.22. Ferrara N. Vascular endothelial growth factor: molecular and abdominal aspects. Curr Top Microbiol Immunol. 1999; 237:1–30.23. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence abdominal findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005; 36:336–9.24. Schaal KB, Höh AE, Scheuerle A, et al. Bevacizumab for the abdominal of macular edema secondary to retinal vein occlusion. Ophthalmologe. 2007; 104:285–9.25. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal abdominal (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006; 26:279–84.26. Lee SW, Kim MS, Kim ES, Kwak HW. Long-Term Results of Intravitreal Bevacizumab Injection for Macular Edema: Retinal Vein Obstruction and Diabetic Retinopathy. J Korean Ophthalmol Soc. 2009; 50:211–8.
Article27. Beutel J, Ziemssen F, Luke M, et al. Intravitreal bevacizumab treatment of macular edema in central retinal vein occlusion: one-year results. Int Ophthalmol. 2010; 30:15–22.
Article28. Ferrara DC, Koizumi H, Spaide RF. Early bevacizumab abdominal of central retinal vein occlusion. Am J Ophthalmol. 2007; 144:864–71.29. Rensch F, Jonas JB, Spandau UH. Early intravitreal bevacizumab for non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2009; 87:77–81.
Article30. Bashshur ZF, Ma'luf RN, Allam S, et al. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1137–40.31. Spaide RF, Chang LK, Klancnik JM, et al. Prospective study of abdominal ranibizumab as a treatment for decreased visual acuity secondary to central retinal vein occlusion. Am J Ophthalmol. 2009; 147:298–306.32. Hsu J, Kaiser RS, Sivalingam A, et al. Intravitreal bevacizumab (avastin) in central retinal vein occlusion. Retina. 2007; 27:1013–9.
Article33. Badala F. The treatment of branch retinal vein occlusion with bevacizumab. Curr Opin Ophthalmol. 2008; 19:234–8.34. Prager F, Michels S, Kriechbaum K, et al. Intravitreal abdominal (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009; 93:452–6.35. Scott IU, Edwards AR, Beck RW, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007; 114:1860–7.
Article36. Matsumoto Y, Freund KB, Peiretti E, et al. Rebound macular edema following bevacizumab (Avastin) therapy for retinal venous occlusive disease. Retina. 2007; 27:426–31.
Article37. Rabena MD, Pieramici DJ, Castellarin AA, et al. Intravitreal abdominal (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2007; 27:419–25.38. Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006; 90:1344–9.
Article39. Regillo CD BG, Flynn HW. Vitreoretinal Disease: The Essentials. New York: Georg Thieme;1999. p. 129.40. Kim KS, Chang HR, Song S. Ischaemic change after intravitreal bevacizumab (Avastin) injection for macular oedema secondary to non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2008; 86:925–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy of Intravitreal Bevacizumab in the Treatment of Macular Edema
- Short-term Effects of Intravitreal Bevacizumab Injection and Macular Edema Patterns in Branch Retinal Vein Occlusion
- Effects of Intravitreal Bevacizumab Injection in 3 Types of Macular Edema Secondary to Branch Retinal Vein Occlusion
- Macular Vessel Density Analysis Using Optical Coherence Tomography Angiography before and after Intravitreal Bevacizumab Injection in Branch Retinal Vein Occlusion
- Intravitreal Bevacizumab Injection for Macular Edema Secondary to Branch Retinal Vein Occlusion