Hip Pelvis.
2017 Jun;29(2):81-90. 10.5371/hp.2017.29.2.81.
Management of Blood Loss in Hip Arthroplasty: Korean Hip Society Current Consensus
- Affiliations
-
- 1Department of Orthopaedics, St. Vincent's Hospital, The Catholic University of Korea, Suwon, Korea.
- 2Department of Orthopaedic Surgery, School of Medicine, Ewha Womans University, Seoul, Korea.
- 3Department of Orthopaedic Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Orthopedic Surgery, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea.
- 5Department of Orthopaedic Surgery, Soonchunhyang University Hospital, Seoul, Korea.
- 6Department of Orthopaedic Surgery, Keimyung University School of Medicine, Daegu, Korea.
- 7Department of Orthopaedic surgery, Yonsei University, College of Medicine, Seoul, Korea.
- 8Department of Orthopaedic Surgery, School of Medicine, Hanyang University, Seoul, Korea.
- 9Department of Orthopaedic surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 10Department of Orthopedic Surgery, Korea University Anam Hospital, Seoul, Korea. oshan@korea.ac.kr
- KMID: 2424235
- DOI: http://doi.org/10.5371/hp.2017.29.2.81
Abstract
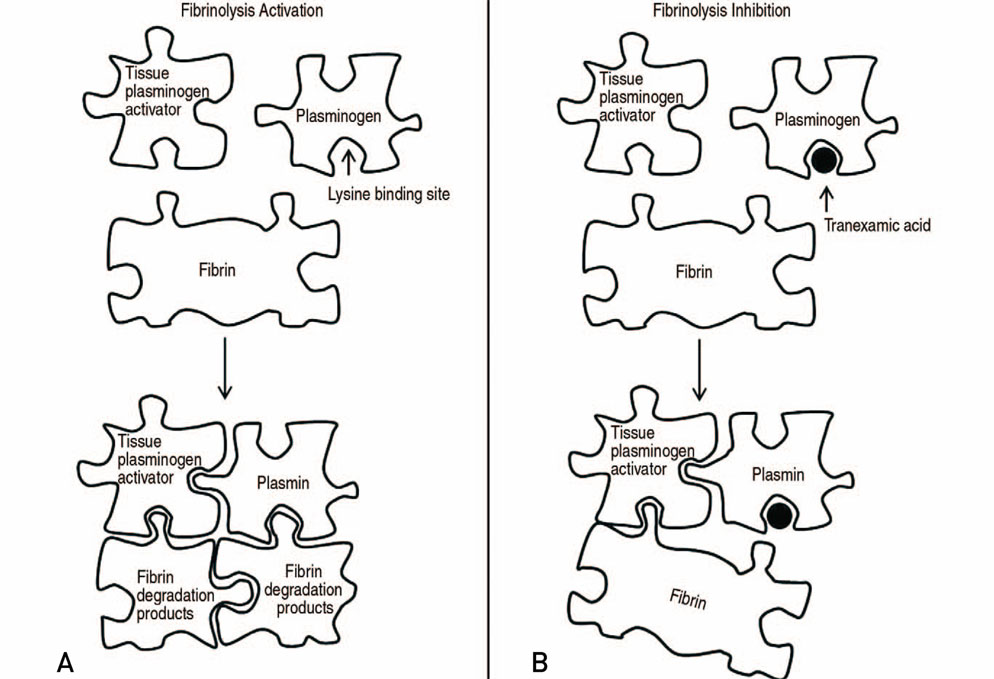
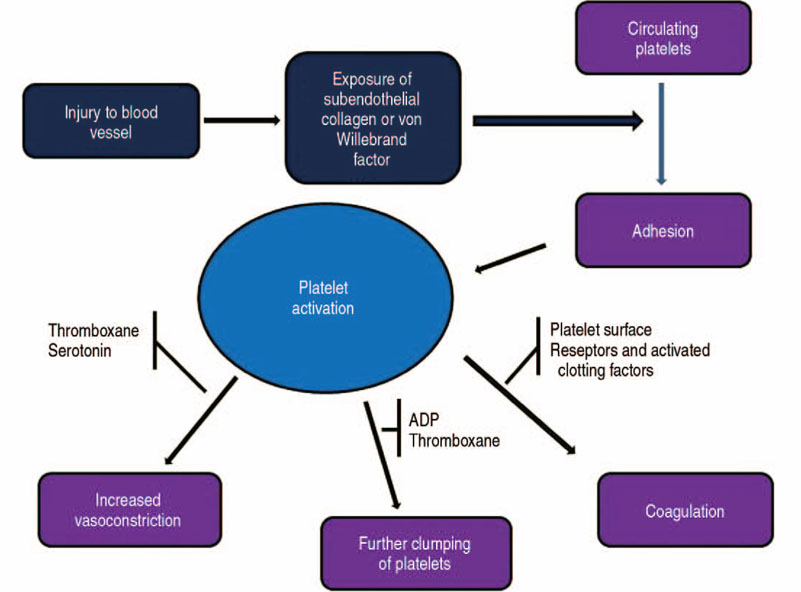
- The volume of hip arthroplasty is stiffly increasing because of excellent clinical outcomes, however it has not been shown to decrease the incidence of transfusions due to bleeding related to this surgery. This is an important consideration since there are concerns about the side effects and social costs of transfusions. First, anemia should be assessed at least 30 days before elective hip arthroplasty, and if the subject is diagnosed as having anemia, an additional examination of the cause of the anemia should be carried and steps taken to address the anemia. Available iron treatments for anemia take 7 to 10 days to facilitate erythropoiesis, and preoperative iron supplementation, either oral or intravenous, is recommended. When using oral supplements for iron storage, administer elemental iron 100 mg daily for 2 to 6 weeks before surgery, and calculate the dose using intravenous supplement. Tranexamic acid (TXA) is a synthetic derivative of the lysine component, which reduces blood loss by inhibiting fibrinolysis and clot degradation. TXA is known to be an effective agent for reducing postoperative bleeding and reducing the need for transfusions in primary and revision total hip arthroplasties. Patient blood management has improved the clinical outcome after hip arthroplasty through the introduction and research of various agents, thereby reducing the need for allogeneic blood transfusions and reducing the risk of transfusion-related infections and the duration of hospitalizations.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
A Paradigm Shift: Perioperative Iron and Erythropoietin Therapy for Patient Blood Management
Hyesun Lee, Young Jin Yuh
Hanyang Med Rev. 2018;38(1):16-26. doi: 10.7599/hmr.2018.38.1.16.Postoperative Intravenous Ferric Carboxymaltose Reduces Transfusion Amounts after Orthopedic Hip Surgery
Sun Ki Kim, Won Yeong Seo, Hee Joong Kim, Jeong Joon Yoo
Clin Orthop Surg. 2018;10(1):20-25. doi: 10.4055/cios.2018.10.1.20.
Reference
-
1. Park JH, Kim HS, Yoo JH, et al. Perioperative blood loss in bipolar hemiarthroplasty for femoral neck fracture: analysis of risk factors. Hip Pelvis. 2013; 25:110–114.
Article2. Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011; 378:1396–1407.
Article3. Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016; 14:23–65.4. Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011; 106:13–22.
Article5. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009; 12:444–454.
Article6. Muñoz M, García-Erce JA, Cuenca J, Bisbe E, Naveira E. AWGE (Spanish Anaemia Working Group). On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012; 10:8–22.7. Foss NB, Kristensen MT, Kehlet H. Anaemia impedes functional mobility after hip fracture surgery. Age Ageing. 2008; 37:173–178.
Article8. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010; 92:265–269.9. Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica. 2014; 99:1671–1676.
Article10. Adamson JW. The relationship of erythropoietin and iron metabolism to red blood cell production in humans. Semin Oncol. 1994; 21:2 Suppl 3. 9–15.11. Goodnough LT. Iron deficiency syndromes and iron-restricted erythropoiesis (CME). Transfusion. 2012; 52:1584–1592.
Article12. Auerbach M, Goodnough LT, Shander A. Iron: the new advances in therapy. Best Pract Res Clin Anaesthesiol. 2013; 27:131–140.
Article13. Baron DM, Hochrieser H, Posch M, et al. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth. 2014; 113:416–423.
Article14. Rosencher N, Poisson D, Albi A, Aperce M, Barré J, Samama CM. Two injections of erythropoietin correct moderate anemia in most patients awaiting orthopedic surgery. Can J Anaesth. 2005; 52:160–165.
Article15. Theusinger OM, Kind SL, Seifert B, Borgeat L, Gerber C, Spahn DR. Patient blood management in orthopaedic surgery: a four-year follow-up of transfusion requirements and blood loss from 2008 to 2011 at the Balgrist University Hospital in Zurich, Switzerland. Blood Transfus. 2014; 12:195–203.16. Theusinger OM, Spahn DR. Perioperative blood conservation strategies for major spine surgery. Best Pract Res Clin Anaesthesiol. 2016; 30:41–52.
Article17. Jetty V, Glueck CJ, Freiberg RA, Wang P. Venous thromboembolism after knee arthroscopy in undiagnosed familial thrombophilia. Orthopedics. 2016; 39:e1052–e1057.
Article18. Moon YW, Kim YS, Kwon SY, Kim SY, Lim SJ, Park YS. Perioperative risk of hip arthroplasty in patients with cirrhotic liver disease. J Korean Med Sci. 2007; 22:223–226.
Article19. Shih LY, Cheng CY, Chang CH, Hsu KY, Hsu RW, Shih HN. Total knee arthroplasty in patients with liver cirrhosis. J Bone Joint Surg Am. 2004; 86-A:335–341.
Article20. Rodríguez-Merchán EC. Total knee arthroplasty in hemophilic arthropathy. Am J Orthop (Belle Mead NJ). 2015; 44:E503–E507.21. Yamada Y, Fujimoto-Ibusuki K, Morikawa-Kubota K. Perioperative management of factor XI deficiency in a patient undergoing hip arthroplasty. J Anesth. 2014; 28:618–620.
Article22. Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013; 30:270–382.23. Bisbe E, Moltó L. Pillar 2: minimising bleeding and blood loss. Best Pract Res Clin Anaesthesiol. 2013; 27:99–110.
Article24. Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008; 108:71–77.
Article25. Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006; 103:1018–1025.
Article26. Richman JM, Rowlingson AJ, Maine DN, Courpas GE, Weller JF, Wu CL. Does neuraxial anesthesia reduce intraoperative blood loss? A meta-analysis. J Clin Anesth. 2006; 18:427–435.27. Haughom BD, Schairer WW, Nwachukwu BU, Hellman MD, Levine BR. Does neuraxial anesthesia decrease transfusion rates following total hip arthroplasty? J Arthroplasty. 2015; 30:9 Suppl. 116–120.
Article28. Rashiq S, Finegan BA. The effect of spinal anesthesia on blood transfusion rate in total joint arthroplasty. Can J Surg. 2006; 49:391–396.29. Suh YS, Nho JH, Choi HS, Ha YC, Park JS, Koo KH. A protocol avoiding allogeneic transfusion in joint arthroplasties. Arch Orthop Trauma Surg. 2016; 136:1213–1226.
Article30. Boettner F, Sculco P, Altneu E, Capar B, Sculco TP. Efficiency of autologous blood donation in combination with a cell saver in bilateral total knee arthroplasty. HSS J. 2009; 5:45–48.
Article31. van Bodegom-Vos L, Voorn VM, So-Osman C, et al. Cell salvage in hip and knee arthroplasty: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2015; 97:1012–1021.32. Shemshaki H, Nourian SM, Nourian N, Dehghani M, Mokhtari M, Mazoochian F. One step closer to sparing total blood loss and transfusion rate in total knee arthroplasty: a meta-analysis of different methods of tranexamic acid administration. Arch Orthop Trauma Surg. 2015; 135:573–588.
Article33. Holt JB, Miller BJ, Callaghan JJ, Clark CR, Willenborg MD, Noiseux NO. Minimizing blood transfusion in total hip and knee arthroplasty through a multimodal approach. J Arthroplasty. 2016; 31:378–382.
Article34. Kamath AF, Pagnano MW. Blood management for patients undergoing total joint arthroplasty. JBJS Rev. Published online Decmber 3, 2013. DOI: 10.2106/JBJS.RVW.M.00046.
Article35. Okamoto S, Sato S, Takada Y, Okamoto U. An active stereo-isomer (trans-form) of AMCHA and its antifibrinolytic (antiplasminic) action in vitro and in vivo. Keio J Med. 1964; 13:177–185.
Article36. Melander B, Gliniecki G, Granstrand B, Hanshoff G. Biochemistry and toxicology of amikapron; the antifibrinolytically active isomer of AMCHA. (A comparative study with epsilon-aminocaproic acid). Acta Pharmacol Toxicol (Copenh). 1965; 22:340–352.
Article37. Petäjä J, Myllynen P, Myllylä G, Vahtera E. Fibrinolysis after application of a pneumatic tourniquet. Acta Chir Scand. 1987; 153:647–651.38. Nadeau RP, Howard JL, Naudie DD. Antifibrinolytic therapy for perioperative blood conservation in lower-extremity primary total joint arthroplasty. JBJS Rev. Published online June 2, 2015. DOI: 10.2106/JBJS.RVW.N.00068.
Article39. Melvin JS, Stryker LS, Sierra RJ. Tranexamic Acid in Hip and Knee Arthroplasty. J Am Acad Orthop Surg. 2015; 23:732–740.
Article40. Loftus TJ, Spratling L, Stone BA, Xiao L, Jacofsky DJ. A patient blood management program in prosthetic joint arthroplasty decreases blood use and improves outcomes. J Arthroplasty. 2016; 31:11–14.
Article41. Kim C, Park SS, Davey JR. Tranexamic acid for the prevention and management of orthopedic surgical hemorrhage: current evidence. J Blood Med. 2015; 6:239–244.42. Hsu CH, Lin PC, Kuo FC, Wang JW. A regime of two intravenous injections of tranexamic acid reduces blood loss in minimally invasive total hip arthroplasty: a prospective randomised double-blind study. Bone Joint J. 2015; 97-B:905–910.43. Gao F, Sun W, Guo W, Li Z, Wang W, Cheng L. Topical application of tranexamic acid plus diluted epinephrine reduces postoperative hidden blood loss in total hip arthroplasty. J Arthroplasty. 2015; 30:2196–2200.
Article44. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013; 95:1969–1974.
Article45. Phillips SJ, Chavan R, Porter ML, et al. Does salvage and tranexamic acid reduce the need for blood transfusion in revision hip surgery? J Bone Joint Surg Br. 2006; 88:1141–1142.
Article46. Noordin S, Waters TS, Garbuz DS, Duncan CP, Masri BA. Tranexamic acid reduces allogenic transfusion in revision hip arthroplasty. Clin Orthop Relat Res. 2011; 469:541–546.
Article47. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011; 93:39–46.
Article48. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014; 96-B:1005–1015.
Article49. Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980; 14:41–47.
Article50. Jenkins SN, Neyman KM, Veledar E, Chen SC. A pilot study evaluating the efficacy of botulinum toxin A in the treatment of Raynaud phenomenon. J Am Acad Dermatol. 2013; 69:834–835.
Article51. Fergusson DA, Hébert PC, Mazer CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008; 358:2319–2331.
Article52. Howe N, Cherpelis B. Obtaining rapid and effective hemostasis: Part II. Electrosurgery in patients with implantable cardiac devices. J Am Acad Dermatol. 2013; 69:677.e1–677.e9.53. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011; 365:2453–2462.
Article54. de Kanter R, Sidharta PN, Delahaye S, et al. Physiologically-based pharmacokinetic modeling of macitentan: prediction of drug-drug interactions. Clin Pharmacokinet. 2016; 55:369–380.
Article55. Ford NF. The metabolism of clopidogrel: CYP2C19 is a minor pathway. J Clin Pharmacol. 2016; 56:1474–1483.
Article56. Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007; 47:1468–1480.
Article57. Lasocki S, Krauspe R, von Heymann C, Mezzacasa A, Chainey S, Spahn DR. PREPARE: the prevalence of perioperative anaemia and need for patient blood management in elective orthopaedic surgery: a multicentre, observational study. Eur J Anaesthesiol. 2015; 32:160–167.58. Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009; 110:574–581.59. Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016; 91:31–38.
Article60. Hoigné R, Breymann C, Künzi UP, Brunner F. [Parenteral iron therapy: problems and possible solutions]. Schweiz Med Wochenschr. 1998; 128:528–535. German.61. Szebeni J, Fishbane S, Hedenus M, et al. Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. Br J Pharmacol. 2015; 172:5025–5036.
Article62. Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M;. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011; 107:477–478.
Article63. Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012; 106:943–952.