Allergy Asthma Immunol Res.
2017 Nov;9(6):509-516. 10.4168/aair.2017.9.6.509.
Association Between Sensitization to Mold and Impaired Pulmonary Function in Children With Asthma
- Affiliations
-
- 1Department of Pediatrics, Korea University College of Medicine, Anam Hospital, Seoul, Korea. yoolina@korea.ac.kr
- 2Allergy Immunology Center, Korea University College of Medicine, Anam Hospital, Seoul, Korea. biokorea@korea.ac.kr
- 3Environmental Health Center, Korea University College of Medicine, Anam Hospital, Seoul, Korea.
- KMID: 2421661
- DOI: http://doi.org/10.4168/aair.2017.9.6.509
Abstract
- PURPOSE
Recent data indicate that sensitization to mold contributes to the severity and persistence of asthma. In this study, we investigated the relationships between sensitization to mold and lung function parameters in children with asthma.
METHODS
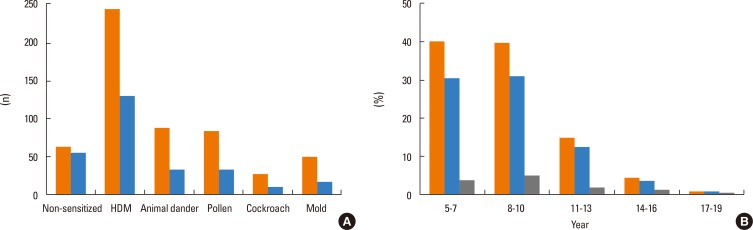
We retrospectively reviewed clinical data from 551 asthmatic subjects. We selected subjects who met clinical diagnostic criteria of asthma. Their spirometry, methacholine challenge tests, and measurements of blood eosinophils, serum IgE, eosinophil cationic protein (ECP) and fractional exhaled nitric oxide (FeNO) results were included. Skin prick testing (SPT) results with 13 common aeroallergens in Korea including house dust mites, animal dander, pollen, cockroach and mold were reviewed. Subjects were divided into 3 groups according to their SPT results. Subjects who showed no positive result to any aeroallergen were designated as group 1 (non-sensitized). Group 2 represented subjects who were sensitized to aeroallergens other than mold (other allergen-sensitized) and group 3 included subjects who were sensitized to mold allergens (mold-sensitized).
RESULTS
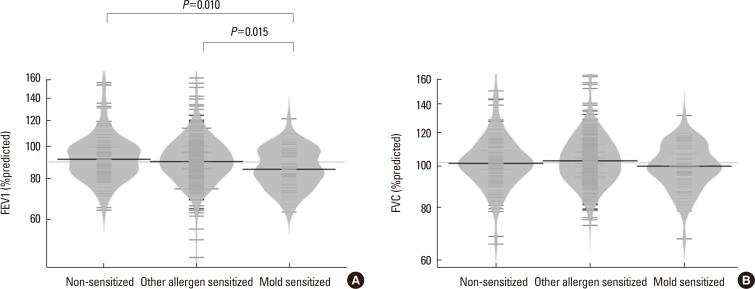
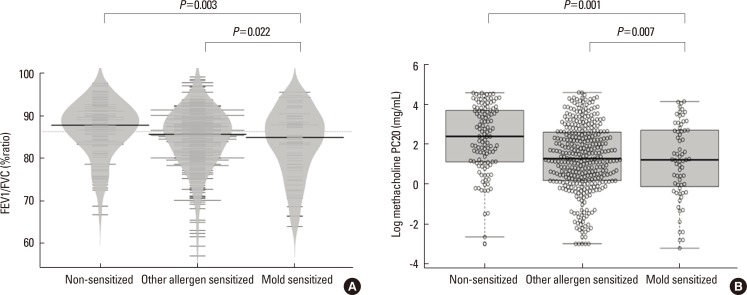
Among the 551 asthmatic subjects, 67 (12.2%) were sensitized to mold and 366 (66.4%) were sensitized to other aeroallergens. The log mean IgE levels were higher in groups 2 (5.96±1.14 IU/mL) and 3 (5.81±0.97 IU/mL) compared to group 1 (3.88±1.68 IU/mL). Blood eosinophils, ECP and FeNO concentrations were significantly higher in groups 2 and 3, but no significant difference was found between the 2 groups. The mean FEV1 value was significantly lower in group 3 (86.9±12.1%pred) than in groups 2 (92.0±14.8%pred) and 1 (93.4±15.4%pred). The log mean methacholine PC20 was significantly lower in group 3 (0.08±1.91 mg/mL) than in groups 2 (1.31±1.69 mg/mL) and 1 (2.29±1.66 mg/mL).
CONCLUSIONS
We observed a differential association between mold and other aeroallergen sensitization, and severity of asthma. Sensitization to mold is associated with lower lung function and increased airway hyper-responsiveness in children with asthma. Mold sensitization could be an important factor determining asthma severity particularly airflow limitation in children.
MeSH Terms
-
Allergens
Animals
Asthma*
Child*
Cockroaches
Dander
Eosinophil Cationic Protein
Eosinophils
Fungi*
Humans
Immunoglobulin E
Korea
Lung
Methacholine Chloride
Nitric Oxide
Pollen
Pyroglyphidae
Respiratory Hypersensitivity
Retrospective Studies
Skin
Spirometry
Allergens
Eosinophil Cationic Protein
Immunoglobulin E
Methacholine Chloride
Nitric Oxide
Figure
Cited by 2 articles
-
Changes of aeroallergen sensitization in childhood
So-Yeon Lee
Allergy Asthma Respir Dis. 2019;7(4):171-172. doi: 10.4168/aard.2019.7.4.171.Recent changing pattern of aeroallergen sensitization in children with allergic diseases: A single center study
Su-Jin Lee, Jung-Min Kim, Hyo-Bin Kim
Allergy Asthma Respir Dis. 2019;7(4):186-191. doi: 10.4168/aard.2019.7.4.186.
Reference
-
1. Gent JF, Kezik JM, Hill ME, Tsai E, Li DW, Leaderer BP. Household mold and dust allergens: exposure, sensitization and childhood asthma morbidity. Environ Res. 2012; 118:86–93. PMID: 22863552.
Article2. Ezeamuzie CI, Al-Ali S, Khan M, Hijazi Z, Dowaisan A, Thomson MS, et al. IgE-mediated sensitization to mould allergens among patients with allergic respiratory diseases in a desert environment. Int Arch Allergy Immunol. 2000; 121:300–307. PMID: 10828720.
Article3. Moreno A, Pineda F, Alcover J, Rodríguez D, Palacios R, Martínez-Naves E. Orthologous allergens and diagnostic utility of major allergen Alt a 1. Allergy Asthma Immunol Res. 2016; 8:428–437. PMID: 27334781.
Article4. Menzies D, Holmes L, McCumesky G, Prys-Picard C, Niven R. Aspergillus sensitization is associated with airflow limitation and bronchiectasis in severe asthma. Allergy. 2011; 66:679–685. PMID: 21261660.5. Vicencio AG, Santiago MT, Tsirilakis K, Stone A, Worgall S, Foley EA, et al. Fungal sensitization in childhood persistent asthma is associated with disease severity. Pediatr Pulmonol. 2014; 49:8–14. PMID: 23401301.
Article6. Niedoszytko M, Chełmińska M, Jassem E, Czestochowska E. Association between sensitization to Aureobasidium pullulans (Pullularia sp) and severity of asthma. Ann Allergy Asthma Immunol. 2007; 98:153–156. PMID: 17304882.7. Plaza V, Serrano J, Picado C, Cosano J, Ancochea J, de Diego A, et al. Clinical characteristics of the fatal and near-fatal asthma in Alternaria alternata sensitized patients. Med Clin (Barc). 2003; 121:721–724. PMID: 14678692.8. Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000; 55:501–504. PMID: 10843433.
Article9. Pongracic JA, O'Connor GT, Muilenberg ML, Vaughn B, Gold DR, Kattan M, et al. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J Allergy Clin Immunol. 2010; 125:593–599. PMID: 20132971.
Article10. O'Hollaren MT, Yunginger JW, Offord KP, Somers MJ, O'Connell EJ, Ballard DJ, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991; 324:359–363. PMID: 1987459.11. Chinn S, Jarvis D, Luczynska C, Burney P. Individual allergens as risk factors for bronchial responsiveness in young adults. Thorax. 1998; 53:662–667. PMID: 9828852.
Article12. Fairs A, Agbetile J, Hargadon B, Bourne M, Monteiro WR, Brightling CE, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010; 182:1362–1368. PMID: 20639442.13. Ma Y, Tian G, Tang F, Yu B, Chen Y, Cui Y, et al. The link between mold sensitivity and asthma severity in a cohort of northern Chinese patients. J Thorac Dis. 2015; 7:585–590. PMID: 25973223.14. Jo EJ, Kim MY, Lee SE, Lee SY, Kim MH, Song WJ, et al. Eosinophilic airway inflammation and airway hyperresponsiveness according to aeroallergen sensitization pattern in patients with lower airway symptoms. Allergy Asthma Immunol Res. 2014; 6:39–46. PMID: 24404392.
Article15. Schwartz J, Weiss ST. Relationship of skin test reactivity to decrements in pulmonary function in children with asthma or frequent wheezing. Am J Respir Crit Care Med. 1995; 152:2176–2180. PMID: 8520794.
Article16. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 92:44–47.17. American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995; 152:1107–1136. PMID: 7663792.18. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, et al. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975; 56:323–327. PMID: 1176724.
Article19. Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res. 2015; 7:205–220. PMID: 25840710.
Article20. Mari A, Schneider P, Wally V, Breitenbach M, Simon-Nobbe B. Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin Exp Allergy. 2003; 33:1429–1438. PMID: 14519151.
Article21. Bartra J, Belmonte J, Torres-Rodriguez JM, Cistero-Bahima A. Sensitization to Alternaria in patients with respiratory allergy. Front Biosci (Landmark Ed). 2009; 14:3372–3379. PMID: 19273281.22. Kim DH, Park YS, Jang HJ, Kim JH, Lim DH. Prevalence and allergen of allergic rhinitis in Korean children. Am J Rhinol Allergy. 2016; 30:72–78. PMID: 27216339.
Article23. Park SH, Lim DH, Son BK, Kim JH, Song YE, Oh IB, et al. Sensitization rates of airborne pollen and mold in children. Korean J Pediatr. 2012; 55:322–329. PMID: 23049589.
Article24. Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol. 2012; 130:639–644.e5. PMID: 22789397.
Article25. Kennedy K, Grimes C. Indoor water and dampness and the health effects on children: a review. Curr Allergy Asthma Rep. 2013; 13:672–680. PMID: 24249387.
Article26. Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ. 2002; 325:411–414. PMID: 12193354.
Article27. Karvala K, Toskala E, Luukkonen R, Lappalainen S, Uitti J, Nordman H. New-onset adult asthma in relation to damp and moldy workplaces. Int Arch Occup Environ Health. 2010; 83:855–865. PMID: 20127354.
Article28. Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med. 1997; 155:1356–1361. PMID: 9105079.29. Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008; 372:1058–1064. PMID: 18805334.
Article30. Yoo Y. Does specific fungal allergen really matter? Allergy Asthma Immunol Res. 2016; 8:389–390. PMID: 27334775.
Article31. Denning DW, O'Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006; 27:615–626. PMID: 16507864.
Article32. Li J, Huang Y, Lin X, Zhao D, Tan G, Wu J, et al. Influence of degree of specific allergic sensitivity on severity of rhinitis and asthma in Chinese allergic patients. Respir Res. 2011; 12:95. PMID: 21831329.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sensitization to House Dust Mite: Its Associations with Bronchial Hyperresponsiveness and Lung Function in Asthmatic Children
- Relationship between Allergen Sensitization and Frequency of Asthma in Preschool Atopic Dermatitis Children
- Seasonality of asthma exacerbation in children caused by respiratory virus infection and allergen sensitization
- Change of Inhalant Allergen Sensitization in Children with Allergic Respiratory Diseases during Recent 10 Years
- Recent changing pattern of aeroallergen sensitization in children with allergic diseases: A single center study