Perinatology.
2018 Sep;29(3):133-137. 10.14734/PN.2018.29.3.133.
Perinatal Stroke Associated with Placental Chorioangioma: A Rare Case and Review of Literature
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ohsymd.oh@samsung.com
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2421579
- DOI: http://doi.org/10.14734/PN.2018.29.3.133
Abstract
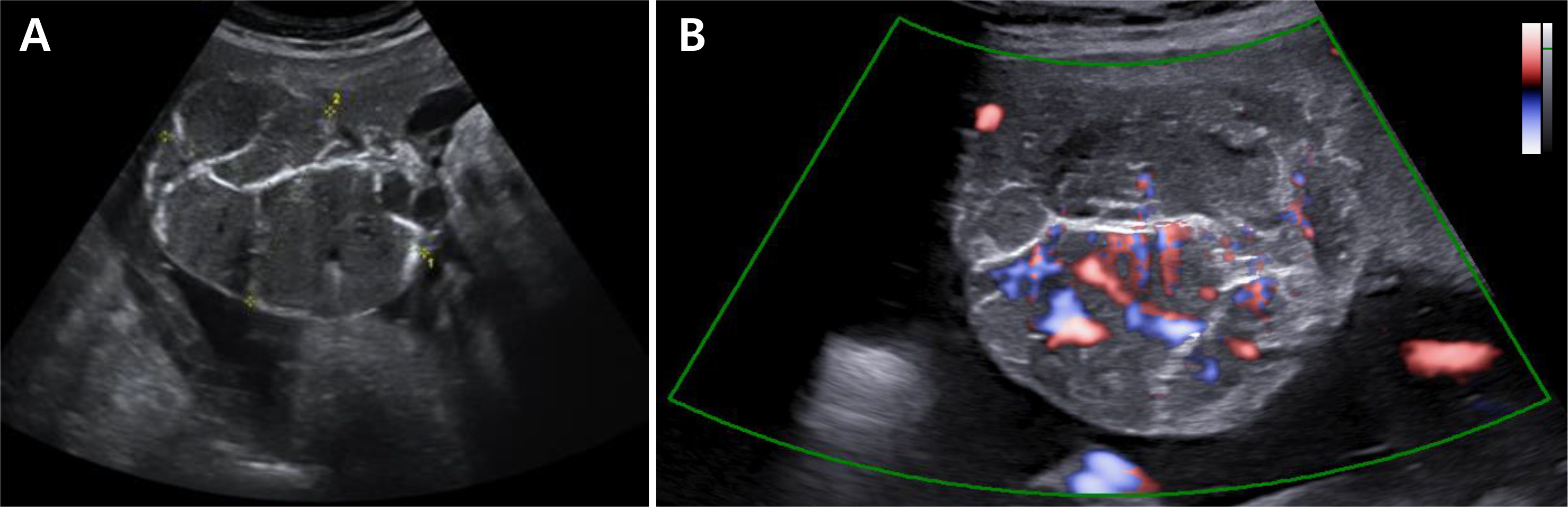
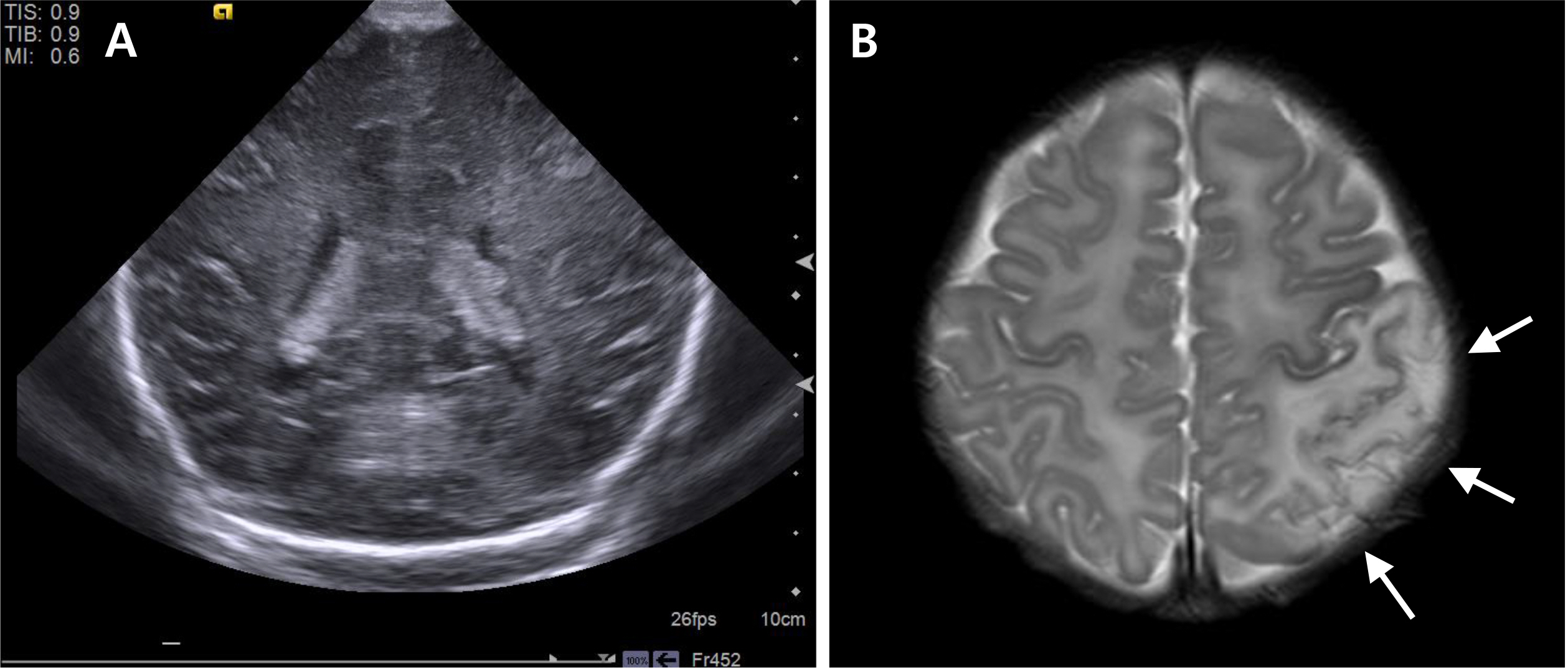
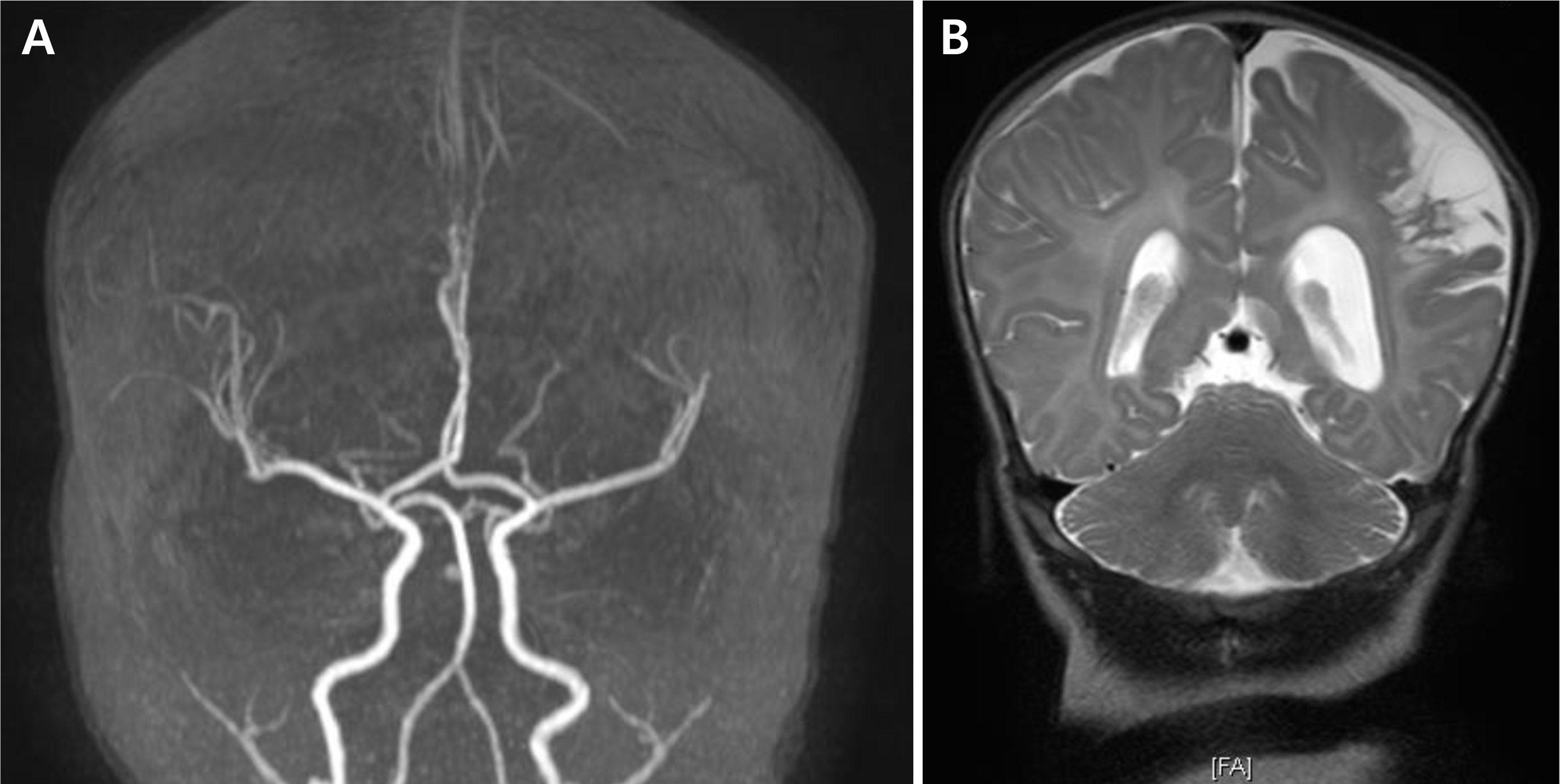
- Placental chorioangioma is the most common non-trophoblastic and hamartoma-like tumor and has generally good prognosis if the size is small. The incidence of a placental chorioangioma is an estimated 1% of all deliveries. The size of a chorioangioma is considered significant if it is larger than 4 cm, since fetal compromise can occur due to circulatory overload. Very rarely, a placental chorioangioma can directly exert fetal circulation to cause fetal cerebral ischemic stroke, especially in giant placental chorioangiomas which are defined as more than 4-5 cm in diameter. Here, we report a case of a huge chorioangioma, sized 7×5 cm with thrombo-occlusion in the placenta associated with neonatal stroke. Perinatal stroke and giant placental chorioangioma are each very rare. Moreover, the combination is extremely rare as is expected. Our case implicates that placental examination should be considered as an important diagnostic workup in cases of perinatal stroke with unknown etiology.
MeSH Terms
Figure
Reference
-
1). Amer HZ., Heller DS. Chorangioma and related vascular lesions of the placenta—a review. Fetal Pediatr Pathol. 2010. 29:199–206.2). Kodandapani S., Shreshta A., Ramkumar V., Rao L. Chorioangioma of placenta: a rare placental cause for adverse fetal outcome. Case Rep Obstet Gynecol. 2012. 2012:913878.
Article3). Wu Z., Hu W. Clinical analysis of 26 patients with histologically proven placental chorioangiomas. Eur J Obstet Gynecol Reprod Biol. 2016. 199:156–63.
Article4). Mineyko A., Kirton A. The black box of perinatal ischemic stroke pathogenesis. J Child Neurol. 2011. 26:1154–62.
Article5). Zanardini C., Papageorghiou A., Bhide A., Thilaganathan B. Giant placental chorioangioma: natural history and pregnancy outcome. Ultrasound Obstet Gynecol. 2010. 35:332–6.
Article6). Li C., Miao JK., Xu Y., Hua YY., Ma Q., Zhou LL, et al. Prenatal, perinatal and neonatal risk factors for perinatal arterial ischaemic stroke: a systematic review and meta-analysis. Eur J Neurol. 2017. 24:1006–15.
Article7). Deeg KH. Sonographic and Doppler sonographic diagnosis of neonatal ischemic stroke. Ultraschall Med. 2017. 38:360–76.8). Ghidini A., Locatelli A. Diffuse placental chorioangiomatosis causing multiple fetal cerebral embolism: a case report. J Reprod Med. 2006. 51:321–4.9). Al Wattar BH., Hillman SC., Marton T., Foster K., Kilby MD. Placenta chorioangioma: a rare case and systematic review of literature. J Matern Fetal Neonatal Med. 2014. 27:1055–63.
Article10). Zanardini C., Papageorghiou A., Bhide A., Thilaganathan B. Giant placental chorioangioma: natural history and pregnancy outcome. Ultrasound Obstet Gynecol. 2010. 35:332–6.
Article11). Das S., Ankola P., Chiechi M., Sandhu J. Perinatal cerebral arterial infarction associated with a placental chorioangioma. Am J Perinatol. 2008. 25:381–3.
Article12). Roh CR. Ischemic perinatal stroke. Korean J Obstet Gynecol. 2009. 52:391–7.13). Lehman LL., Rivkin MJ. Perinatal arterial ischemic stroke: presentation, risk factors, evaluation, and outcome. Pediatr Neurol. 2014. 51:760–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Placental Chorioangioma Combined with Non-immune Hydrops Fetalis
- A Case of Multiple Placental Chorioangioma Combined with Oligohydramnios
- Clinical Significance of Large Placental Chorioangioma
- A case of chorioangioma of placenta complicated by fetal anemia and fetal hydrops
- A Case of Hydrops Fetalis due to Placental Chorangiomatosis