Endocrinol Metab.
2018 Jun;33(2):175-184. 10.3803/EnM.2018.33.2.175.
Genome-Wide Association Studies of Autoimmune Thyroid Diseases, Thyroid Function, and Thyroid Cancer
- Affiliations
-
- 1Center for Thyroid Cancer, National Cancer Center, Goyang, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. yjparkmd@snu.ac.kr
- KMID: 2420484
- DOI: http://doi.org/10.3803/EnM.2018.33.2.175
Abstract
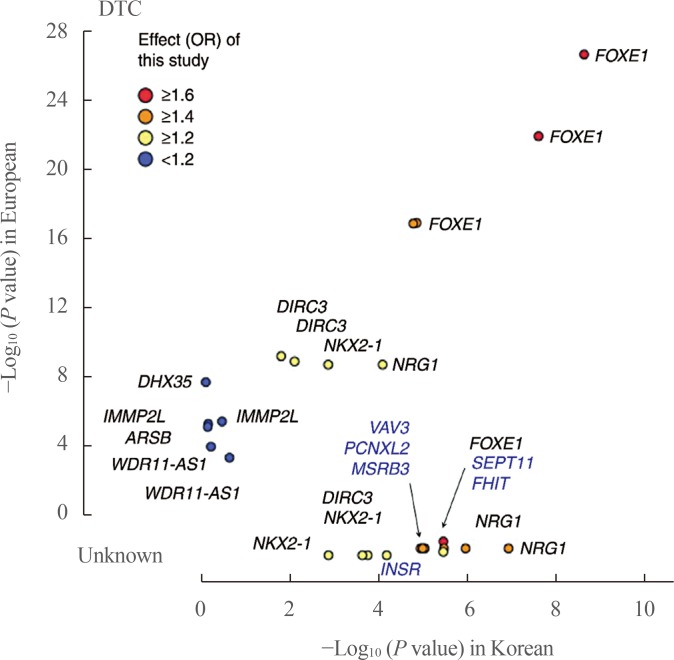
- Thyroid diseases, including autoimmune thyroid diseases and thyroid cancer, are known to have high heritability. Family and twin studies have indicated that genetics plays a major role in the development of thyroid diseases. Thyroid function, represented by thyroid stimulating hormone (TSH) and free thyroxine (T4), is also known to be partly genetically determined. Before the era of genome-wide association studies (GWAS), the ability to identify genes responsible for susceptibility to thyroid disease was limited. Over the past decade, GWAS have been used to identify genes involved in many complex diseases, including various phenotypes of the thyroid gland. In GWAS of autoimmune thyroid diseases, many susceptibility loci associated with autoimmunity (human leukocyte antigen [HLA], protein tyrosine phosphatase, non-receptor type 22 [PTPN22], cytotoxic T-lymphocyte associated protein 4 [CTLA4], and interleukin 2 receptor subunit alpha [IL2RA]) or thyroid-specific genes (thyroid stimulating hormone receptor [TSHR] and forkhead box E1 [FOXE1]) have been identified. Regarding thyroid function, many susceptibility loci for levels of TSH and free T4 have been identified through genome-wide analyses. In GWAS of differentiated thyroid cancer, associations at FOXE1, MAP3K12 binding inhibitory protein 1 (MBIP)-NK2 homeobox 1 (NKX2-1), disrupted in renal carcinoma 3 (DIRC3), neuregulin 1 (NRG1), and pecanex-like 2 (PCNXL2) have been commonly identified in people of European and Korean ancestry, and many other susceptibility loci have been found in specific populations. Through GWAS of various thyroid-related phenotypes, many susceptibility loci have been found, providing insights into the pathogenesis of thyroid diseases and disease co-clustering within families and individuals.
Keyword
MeSH Terms
-
Autoimmunity
Genes, Homeobox
Genetics
Genome-Wide Association Study*
Graves Disease
Hashimoto Disease
Humans
Leukocytes
Neuregulin-1
Phenotype
Protein Tyrosine Phosphatase, Non-Receptor Type 22
Receptors, Interleukin-2
T-Lymphocytes, Cytotoxic
Thyroid Diseases*
Thyroid Gland*
Thyroid Neoplasms*
Thyrotropin
Thyroxine
Neuregulin-1
Protein Tyrosine Phosphatase, Non-Receptor Type 22
Receptors, Interleukin-2
Thyrotropin
Thyroxine
Figure
Cited by 1 articles
-
Programmed Cell Death-Ligand 1 (
PD-L1 ) gene Single Nucleotide Polymorphism in Graves’ Disease and Hashimoto’s Thyroiditis in Korean Patients
Jee Hee Yoon, Min-ho Shin, Hee Nam Kim, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
Endocrinol Metab. 2021;36(3):599-606. doi: 10.3803/EnM.2021.965.
Reference
-
1. Vaidya B, Kendall-Taylor P, Pearce SH. The genetics of autoimmune thyroid disease. J Clin Endocrinol Metab. 2002; 87:5385–5397. PMID: 12466323.
Article2. Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer. 2002; 99:260–266. PMID: 11979442.
Article3. Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001; 86:930–934. PMID: 11158069.
Article4. Brix TH, Kyvik KO, Hegedus L. A population-based study of chronic autoimmune hypothyroidism in Danish twins. J Clin Endocrinol Metab. 2000; 85:536–539. PMID: 10690851.
Article5. Dittmar M, Libich C, Brenzel T, Kahaly GJ. Increased familial clustering of autoimmune thyroid diseases. Horm Metab Res. 2011; 43:200–204. PMID: 21287436.
Article6. Tamai H, Ohsako N, Takeno K, Fukino O, Takahashi H, Kuma K, et al. Changes in thyroid function in euthyroid subjects with a family history of Graves' disease: a follow-up study of 69 patients. J Clin Endocrinol Metab. 1980; 51:1123–1127. PMID: 6774999.7. Hemminki K, Li X, Sundquist J, Sundquist K. The epidemiology of Graves' disease: evidence of a genetic and an environmental contribution. J Autoimmun. 2010; 34:J307–J313. PMID: 20056533.
Article8. Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid. 2003; 13:761–764. PMID: 14558919.
Article9. Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002; 87:1068–1072. PMID: 11889165.10. Hansen PS, Brix TH, Sorensen TI, Kyvik KO, Hegedus L. Major genetic influence on the regulation of the pituitary-thyroid axis: a study of healthy Danish twins. J Clin Endocrinol Metab. 2004; 89:1181–1187. PMID: 15001606.
Article11. Panicker V, Wilson SG, Spector TD, Brown SJ, Falchi M, Richards JB, et al. Heritability of serum TSH, free T4 and free T3 concentrations: a study of a large UK twin cohort. Clin Endocrinol (Oxf). 2008; 68:652–659. PMID: 17970774.
Article12. Samollow PB, Perez G, Kammerer CM, Finegold D, Zwartjes PW, Havill LM, et al. Genetic and environmental influences on thyroid hormone variation in Mexican Americans. J Clin Endocrinol Metab. 2004; 89:3276–3284. PMID: 15240603.
Article13. Park YJ, Ahn HY, Choi HS, Kim KW, Park DJ, Cho BY. The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid. 2012; 22:356–362. PMID: 22280228.
Article14. Capezzone M, Marchisotta S, Cantara S, Busonero G, Brilli L, Pazaitou-Panayiotou K, et al. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Relat Cancer. 2008; 15:1075–1081. PMID: 18832444.
Article15. Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, et al. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009; 145:100–105. PMID: 19081481.
Article16. Loh KC. Familial nonmedullary thyroid carcinoma: a meta-review of case series. Thyroid. 1997; 7:107–113. PMID: 9086578.
Article17. Uchino S, Noguchi S, Kawamoto H, Yamashita H, Watanabe S, Yamashita H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002; 26:897–902. PMID: 11965446.
Article18. Peiling Yang S, Ngeow J. Familial non-medullary thyroid cancer: unraveling the genetic maze. Endocr Relat Cancer. 2016; 23:R577–R595. PMID: 27807061.
Article19. Panicker V. Genetics of thyroid function and disease. Clin Biochem Rev. 2011; 32:165–175. PMID: 22147956.20. Wellcome Trust. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007; 447:661–678. PMID: 17554300.
Article21. Simmonds MJ, Howson JM, Heward JM, Cordell HJ, Foxall H, Carr-Smith J, et al. Regression mapping of association between the human leukocyte antigen region and Graves disease. Am J Hum Genet. 2005; 76:157–163. PMID: 15558498.
Article22. Simmonds MJ, Howson JM, Heward JM, Carr-Smith J, Franklyn JA, Todd JA, et al. A novel and major association of HLA-C in Graves' disease that eclipses the classical HLA-DRB1 effect. Hum Mol Genet. 2007; 16:2149–2153. PMID: 17597093.
Article23. Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003; 423:506–511. PMID: 12724780.24. Brand OJ, Barrett JC, Simmonds MJ, Newby PR, McCabe CJ, Bruce CK, et al. Association of the thyroid stimulating hormone receptor gene (TSHR) with Graves' disease. Hum Mol Genet. 2009; 18:1704–1713. PMID: 19244275.
Article25. Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves' disease. J Clin Endocrinol Metab. 2004; 89:5862–5865. PMID: 15531553.
Article26. Wellcome Trust Case Control Consortium. Australo-Anglo-American Spondylitis Consortium (TASC). Burton PR, Clayton DG, Cardon LR, Craddock N, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007; 39:1329–1337. PMID: 17952073.
Article27. Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, et al. A genome-wide association study identifies two new risk loci for Graves' disease. Nat Genet. 2011; 43:897–901. PMID: 21841780.
Article28. Cooper JD, Simmonds MJ, Walker NM, Burren O, Brand OJ, Guo H, et al. Seven newly identified loci for autoimmune thyroid disease. Hum Mol Genet. 2012; 21:5202–5208. PMID: 22922229.
Article29. Denny JC, Crawford DC, Ritchie MD, Bielinski SJ, Basford MA, Bradford Y, et al. Variants near FOXE1 are associated with hypothyroidism and other thyroid conditions: using electronic medical records for genome- and phenome-wide studies. Am J Hum Genet. 2011; 89:529–542. PMID: 21981779.
Article30. Eriksson N, Tung JY, Kiefer AK, Hinds DA, Francke U, Mountain JL, et al. Novel associations for hypothyroidism include known autoimmune risk loci. PLoS One. 2012; 7:e34442. PMID: 22493691.
Article31. Zhao SX, Xue LQ, Liu W, Gu ZH, Pan CM, Yang SY, et al. Robust evidence for five new Graves' disease risk loci from a staged genome-wide association analysis. Hum Mol Genet. 2013; 22:3347–3362. PMID: 23612905.
Article32. Chu X, Shen M, Xie F, Miao XJ, Shou WH, Liu L, et al. An X chromosome-wide association analysis identifies variants in GPR174 as a risk factor for Graves' disease. J Med Genet. 2013; 50:479–485. PMID: 23667180.33. Kwak SH, Park YJ, Go MJ, Lee KE, Kim SJ, Choi HS, et al. A genome-wide association study on thyroid function and anti-thyroid peroxidase antibodies in Koreans. Hum Mol Genet. 2014; 23:4433–4442. PMID: 24722205.
Article34. Medici M, Porcu E, Pistis G, Teumer A, Brown SJ, Jensen RA, et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014; 10:e1004123. PMID: 24586183.35. Matana A, Popovic M, Boutin T, Torlak V, Brdar D, Gunjaca I, et al. Genome-wide meta-analysis identifies novel gender specific loci associated with thyroid antibodies level in Croatians. Genomics. 2018; 4. 18. [Epub]. DOI: 10.1016/j.ygeno.2018.04.012.
Article36. Oryoji D, Ueda S, Yamamoto K, Yoshimura Noh J, Okamura K, Noda M, et al. Identification of a Hashimoto thyroiditis susceptibility locus via a genome-wide comparison with Graves' disease. J Clin Endocrinol Metab. 2015; 100:E319–E324. PMID: 25429627.
Article37. Simmonds MJ. GWAS in autoimmune thyroid disease: redefining our understanding of pathogenesis. Nat Rev Endocrinol. 2013; 9:277–287. PMID: 23529038.
Article38. Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013; 9:e1003266. PMID: 23408906.
Article39. Zhan M, Chen G, Pan CM, Gu ZH, Zhao SX, Liu W, et al. Genome-wide association study identifies a novel susceptibility gene for serum TSH levels in Chinese populations. Hum Mol Genet. 2014; 23:5505–5517. PMID: 24852370.
Article40. Taylor PN, Porcu E, Chew S, Campbell PJ, Traglia M, Brown SJ, et al. Whole-genome sequence-based analysis of thyroid function. Nat Commun. 2015; 6:5681. PMID: 25743335.
Article41. Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet. 2009; 41:460–464. PMID: 19198613.
Article42. Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, He H, et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat Genet. 2012; 44:319–322. PMID: 22267200.
Article43. Kohler A, Chen B, Gemignani F, Elisei R, Romei C, Figlioli G, et al. Genome-wide association study on differentiated thyroid cancer. J Clin Endocrinol Metab. 2013; 98:E1674–E1681. PMID: 23894154.44. Figlioli G, Kohler A, Chen B, Elisei R, Romei C, Cipollini M, et al. Novel genome-wide association study-based candidate loci for differentiated thyroid cancer risk. J Clin Endocrinol Metab. 2014; 99:E2084–E2092. PMID: 25029422.
Article45. Figlioli G, Chen B, Elisei R, Romei C, Campo C, Cipollini M, et al. Novel genetic variants in differentiated thyroid cancer and assessment of the cumulative risk. Sci Rep. 2015; 5:8922. PMID: 25753578.
Article46. Mancikova V, Cruz R, Inglada-Perez L, Fernandez-Rozadilla C, Landa I, Cameselle-Teijeiro J, et al. Thyroid cancer GWAS identifies 10q26.12 and 6q14.1 as novel susceptibility loci and reveals genetic heterogeneity among populations. Int J Cancer. 2015; 137:1870–1878. PMID: 25855579.
Article47. Gudmundsson J, Thorleifsson G, Sigurdsson JK, Stefansdottir L, Jonasson JG, Gudjonsson SA, et al. A genome-wide association study yields five novel thyroid cancer risk loci. Nat Commun. 2017; 8:14517. PMID: 28195142.
Article48. Malinowski JR, Denny JC, Bielinski SJ, Basford MA, Bradford Y, Peissig PL, et al. Genetic variants associated with serum thyroid stimulating hormone (TSH) levels in European Americans and African Americans from the eMERGE Network. PLoS One. 2014; 9:e111301. PMID: 25436638.
Article49. Rawal R, Teumer A, Volzke H, Wallaschofski H, Ittermann T, Asvold BO, et al. Meta-analysis of two genome-wide association studies identifies four genetic loci associated with thyroid function. Hum Mol Genet. 2012; 21:3275–3282. PMID: 22494929.
Article50. Takahashi M, Saenko VA, Rogounovitch TI, Kawaguchi T, Drozd VM, Takigawa-Imamura H, et al. The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Hum Mol Genet. 2010; 19:2516–2523. PMID: 20350937.
Article51. Landa I, Ruiz-Llorente S, Montero-Conde C, Inglada-Perez L, Schiavi F, Leskela S, et al. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet. 2009; 5:e1000637. PMID: 19730683.
Article52. Jendrzejewski J, Liyanarachchi S, Nagy R, Senter L, Wakely PE, Thomas A, et al. Papillary thyroid carcinoma: association between germline DNA variant markers and clinical parameters. Thyroid. 2016; 26:1276–1284. PMID: 27342578.
Article53. Penna-Martinez M, Epp F, Kahles H, Ramos-Lopez E, Hinsch N, Hansmann ML, et al. FOXE1 association with differentiated thyroid cancer and its progression. Thyroid. 2014; 24:845–851. PMID: 24325646.
Article54. Son HY, Hwangbo Y, Yoo SK, Im SW, Yang SD, Kwak SJ, et al. Genome-wide association and expression quantitative trait loci studies identify multiple susceptibility loci for thyroid cancer. Nat Commun. 2017; 8:15966. PMID: 28703219.
Article55. He H, Li W, Liyanarachchi S, Wang Y, Yu L, Genutis LK, et al. The role of NRG1 in the predisposition to papillary thyroid carcinoma. J Clin Endocrinol Metab. 2018; 103:1369–1379. PMID: 29121253.
Article56. Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013; 3:520–533. PMID: 23365119.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preoperative Staging of Well Differentiated Thyroid Cancer: US Is Enough?
- Establishment of Korean Thyroid Association-10 Years of Development in Internal Medicine
- The Management of Thyroid Disease in COVID-19 Pandemic
- Recent Progress in Research On Autoimmune Thyroid Diseases
- Giant Thyroid Cancer in Elderly Patient