Korean J Physiol Pharmacol.
2018 Sep;22(5):555-566. 10.4196/kjpp.2018.22.5.555.
Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Chung-Ang University, Seoul 06973, Korea. pugokjebi@gmail.com, beomjoon74@gmail.com
- 2Department of Medicine, Graduate School, Chung-Ang University, Seoul 06973, Korea.
- 3Biomedical Research Institute, R&D Center, MEDIPOST Co., Ltd., Seongnam 13494, Korea.
- 4Thema Dermatologic Clinic, Seoul 06524, Korea.
- 5Department of Dermatology, Dankook Medical College, Cheonan 31116, Korea.
- 6Fort Hays State University, Hays, KS 67601, USA.
- KMID: 2419005
- DOI: http://doi.org/10.4196/kjpp.2018.22.5.555
Abstract
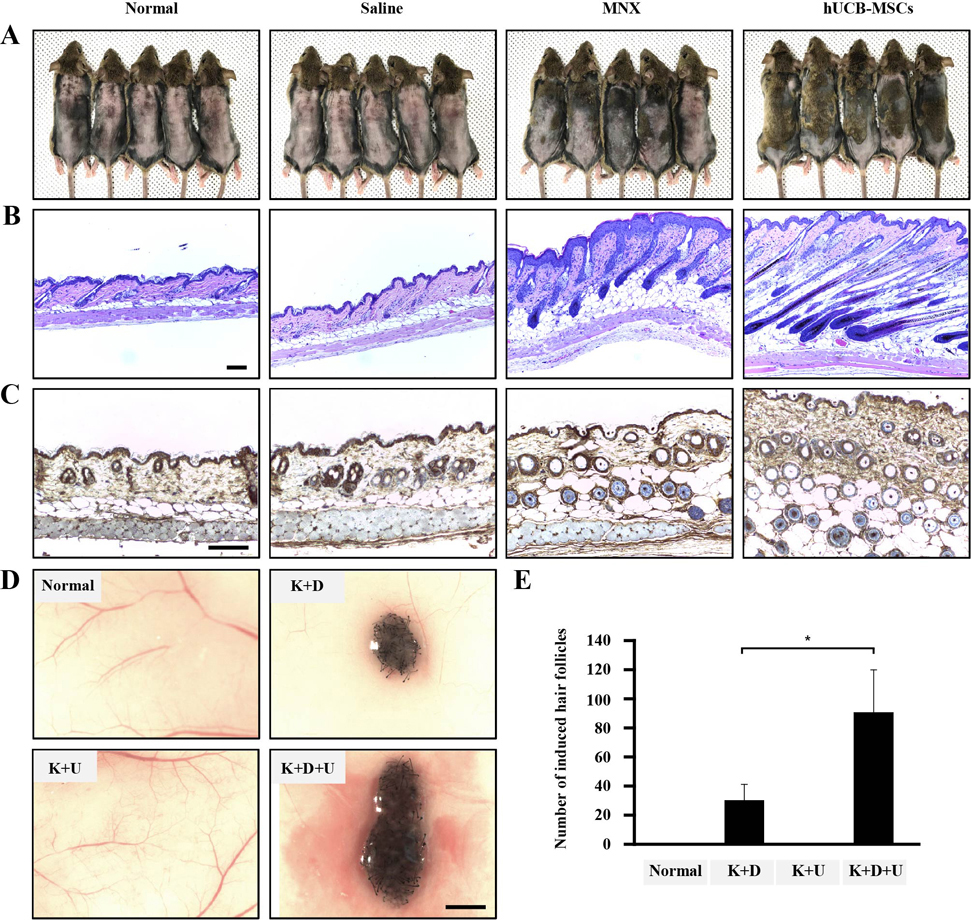
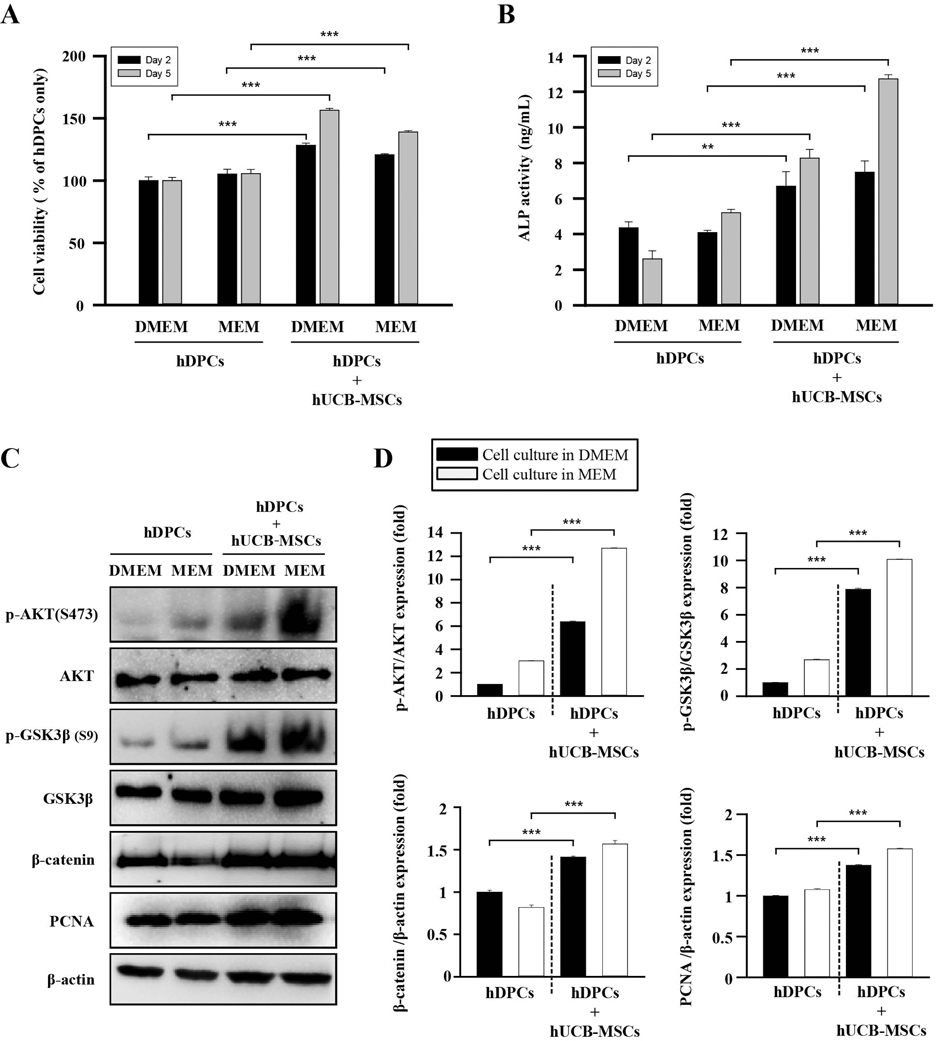
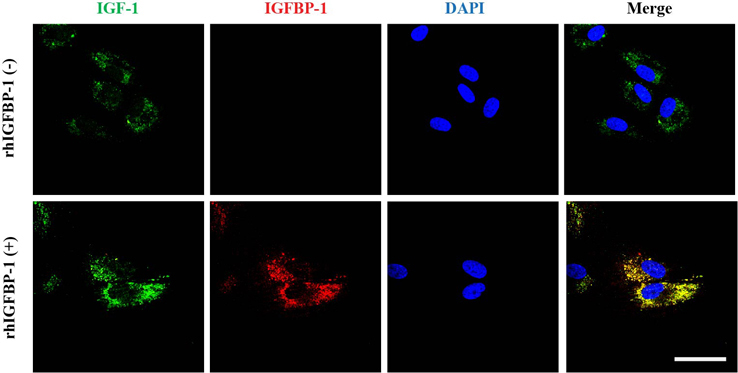
- Human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) are used in tissue repair and regeneration; however, the mechanisms involved are not well understood. We investigated the hair growth-promoting effects of hUCB-MSCs treatment to determine whether hUCB-MSCs enhance the promotion of hair growth. Furthermore, we attempted to identify the factors responsible for hair growth. The effects of hUCB-MSCs on hair growth were investigated in vivo, and hUCB-MSCs advanced anagen onset and hair follicle neogeneration. We found that hUCB-MSCs co-culture increased the viability and up-regulated hair induction-related proteins of human dermal papilla cells (hDPCs) in vitro. A growth factor antibody array revealed that secretory factors from hUCB-MSCs are related to hair growth. Insulin-like growth factor binding protein-1 (IGFBP-1) and vascular endothelial growth factor (VEGF) were increased in co-culture medium. Finally, we found that IGFBP-1, through the co-localization of an IGF-1 and IGFBP-1, had positive effects on cell viability; VEGF secretion; expression of alkaline phosphatase (ALP), CD133, and β-catenin; and formation of hDPCs 3D spheroids. Taken together, these data suggest that hUCB-MSCs promote hair growth via a paracrine mechanism.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Alopecia
Cell Survival
Coculture Techniques
Fetal Blood*
Hair Follicle
Hair*
Humans*
In Vitro Techniques
Insulin-Like Growth Factor Binding Protein 1
Insulin-Like Growth Factor I
Intercellular Signaling Peptides and Proteins*
Mesenchymal Stromal Cells
Regeneration
Stem Cells*
Umbilical Cord*
Vascular Endothelial Growth Factor A
Alkaline Phosphatase
Insulin-Like Growth Factor Binding Protein 1
Insulin-Like Growth Factor I
Intercellular Signaling Peptides and Proteins
Vascular Endothelial Growth Factor A
Figure
Cited by 1 articles
-
The physiological and pharmacological roles of prostaglandins in hair growth
Dong Wook Shin
Korean J Physiol Pharmacol. 2022;26(6):405-413. doi: 10.4196/kjpp.2022.26.6.405.
Reference
-
1. Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961; 21:1440–1447.
Article2. Harada T, Izaki S, Tsutsumi H, Kobayashi M, Kitamura K. Apoptosis of hair follicle cells in the second-degree burn wound unders hypernatremic conditions. Burns. 1998; 24:464–469.
Article3. Leyden J, Dunlap F, Miller B, Winters P, Lebwohl M, Hecker D, Kraus S, Baldwin H, Shalita A, Draelos Z, Markou M, Thiboutot D, Rapaport M, Kang S, Kelly T, Pariser D, Webster G, Hordinsky M, Rietschel R, Katz HI, Terranella L, Best S, Round E, Waldstreicher J. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999; 40:930–937.
Article4. Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998; 317:865–869.5. Kligman AM. Pathologic dynamics of human hair loss. I. Telogen effuvium. Arch Dermatol. 1961; 83:175–198.6. Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008; 59:547–566.
Article7. Leavitt M, Perez-Meza D, Rao NA, Barusco M, Kaufman KD, Ziering C. Effects of finasteride (1 mg) on hair transplant. Dermatol Surg. 2005; 31:1268–1276.
Article8. Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012; 6:130–136.
Article9. Irwig MS, Kolukula S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. 2011; 8:1747–1753.
Article10. Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006; 55:1014–1023.11. Patwardhan N, Mysore V. Hair transplantation: standard guidelines of care. Indian J Dermatol Venereol Leprol. 2008; 74:Suppl. S46–S53.12. Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006; 119:391–393.
Article13. Chueh SC, Lin SJ, Chen CC, Lei M, Wang LM, Widelitz R, Hughes MW, Jiang TX, Chuong CM. Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin Biol Ther. 2013; 13:377–391.
Article14. Harkey MR. Anatomy and physiology of hair. Forensic Sci Int. 1993; 63:9–18.
Article15. Elliott K, Stephenson TJ, Messenger AG. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: implications for the control of hair follicle size and androgen responses. J Invest Dermatol. 1999; 113:873–877.
Article16. Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, Kim JC. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008; 128:262–269.
Article17. Dallob AL, Sadick NS, Unger W, Lipert S, Geissler LA, Gregoire SL, Nguyen HH, Moore EC, Tanaka WK. The effect of finasteride, a 5 alpha-reductase inhibitor, on scalp skin testosterone and dihydrotestosterone concentrations in patients with male pattern baldness. J Clin Endocrinol Metab. 1994; 79:703–706.
Article18. El-Badri NS, Hakki A, Saporta S, Liang X, Madhusodanan S, Willing AE, Sanberg CD, Sanberg PR. Cord blood mesenchymal stem cells: Potential use in neurological disorders. Stem Cells Dev. 2006; 15:497–506.
Article19. Chung JY, Song M, Ha CW, Kim JA, Lee CH, Park YB. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther. 2014; 5:39.
Article20. Kim JY, Kim DH, Kim JH, Yang YS, Oh W, Lee EH, Chang JW. Umbilical cord blood mesenchymal stem cells protect amyloid-β42 neurotoxicity via paracrine. World J Stem Cells. 2012; 4:110–116.21. Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008; 112:295–307.22. Seo Y, Yang SR, Jee MK, Joo EK, Roh KH, Seo MS, Han TH, Lee SY, Ryu PD, Jung JW, Seo KW, Kang SK, Kang KS. Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transplant. 2011; 20:1033–1047.
Article23. Li XY, Zheng ZH, Li XY, Guo J, Zhang Y, Li H, Wang YW, Ren J, Wu ZB. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr Pharm Des. 2013; 19:4893–4899.
Article24. Lee MJ, Yoon TG, Kang M, Kim HJ, Kang KS. Effect of subcutaneous treatment with human umbilical cord blood-derived multipotent stem cells on peripheral neuropathic pain in rats. Korean J Physiol Pharmacol. 2017; 21:153–160.
Article25. Park HW, Kim Y, Chang JW, Yang YS, Oh W, Lee JM, Park HR, Kim DG, Paek SH. Effect of single and double administration of human umbilical cord blood-derived mesenchymal stem cells following focal cerebral ischemia in rats. Exp Neurobiol. 2017; 26:55–65.
Article26. Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, Choi S, Oh W, Yang YS. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004; 6:476–486.
Article27. Olsen EA. Hair disorders. In : Irvine AD, Hoeger PH, Yan AC, editors. Harper's textbook of pediatric dermatology. 3rd ed. 2011. Volume 1, 2:p. 148.1–148.35.28. Mounsey AL, Reed SW. Diagnosing and treating hair loss. Am Fam Physician. 2009; 80:356–362.29. Cotsarelis G, Millar SE. Towards a molecular understanding of hair loss and its treatment. Trends Mol Med. 2001; 7:293–301.
Article30. Semenova E, Koegel H, Hasse S, Klatte JE, Slonimsky E, Bilbao D, Paus R, Werner S, Rosenthal N. Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice. Am J Pathol. 2008; 173:1295–1310.
Article31. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012; 166:1035–1042.
Article32. Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci. 2004; 25:363–373.
Article33. Armstrong DD, Wong VL, Esser KA. Expression of beta-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol Cell Physiol. 2006; 291:C185–C188.34. Dong L, Hao H, Xia L, Liu J, Ti D, Tong C, Hou Q, Han Q, Zhao Y, Liu H, Fu X, Han W. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci Rep. 2014; 4:5432.
Article35. Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005; 124:867–876.
Article36. Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012; 132:237–239.
Article37. Kwon TR, Oh CT, Choi EJ, Park HM, Han HJ, Ji HJ, Kim BJ. Human placental extract exerts hair growth-promoting effects through the GSK-3β signaling pathway in human dermal papilla cells. Int J Mol Med. 2015; 36:1088–1096.
Article38. Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ. 2007; 49:185–195.
Article39. Lin TC, Yen JM, Gong KB, Hsu TT, Chen LR. IGF-1/IGFBP-1 increases blastocyst formation and total blastocyst cell number in mouse embryo culture and facilitates the establishment of a stem-cell line. BMC Cell Biol. 2003; 4:14.40. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995; 16:3–34.
Article41. Baxter RC. Insulin-like growth factor (IGF) binding proteins: the role of serum IGFBPs in regulating IGF availability. Acta Paediatr Scand Suppl. 1991; 372:107–114.
Article42. Shapiro J, Madani S. Alopecia areata: diagnosis and management. Int J Dermatol. 1999; 38:Suppl 1. 19–24.
Article43. Rushton DH, Norris MJ, Dover R, Busuttil N. Causes of hair loss and the developments in hair rejuvenation. Int J Cosmet Sci. 2002; 24:17–23.
Article44. Iguchi M, Hara M, Manome H, Kobayasi H, Tagami H, Aiba S. Communication network in the follicular papilla and connective tissue sheath through gap junctions in human hair follicles. Exp Dermatol. 2003; 12:283–288.
Article45. Hibino T, Nishiyama T. Role of TGF-beta2 in the human hair cycle. J Dermatol Sci. 2004; 35:9–18.46. Messenger AG. The control of hair growth: an overview. J Invest Dermatol. 1993; 101:1 Suppl. 4S–9S.
Article47. Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007; 6:2884–2889.
Article48. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005; 33:1402–1416.
Article49. Anderson DG, Markova D, An HS, Chee A, Enomoto-Iwamoto M, Markov V, Saitta B, Shi P, Gupta C, Zhang Y. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cell source for disc repair. Am J Phys Med Rehabil. 2013; 92:420–429.50. Liu GP, Li YL, Sun J, Cui L, Zhang WJ, Cao YL. Repair of calvarial defects with human umbilical cord blood derived mesenchymal stem cells and demineralized bone matrix in athymic rats. Zhonghua Zheng Xing Wai Ke Za Zhi. 2010; 26:34–38.51. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008; 3:e1886.
Article52. Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007; 30:97–104.
Article53. Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, Si Y, Guo Y, Zang L, Mu Y, Han W. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016; 34:627–639.
Article54. Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009; 218:237–245.
Article55. Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006; 103:8155–8160.
Article56. Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007; 27:2684–2690.
Article57. Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997; 21:355–359.
Article58. Weger N, Schlake T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J Invest Dermatol. 2005; 125:873–882.
Article59. Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, Irié T, Tachikawa T, Sato A, Takeda A, Tsuji T. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012; 3:784.
Article60. Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008; 22:543–557.
Article61. Zhou L, Yang K, Xu M, Andl T, Millar SE, Boyce S, Zhang Y. Activating β-catenin signaling in CD133-positive dermal papilla cells increases hair inductivity. FEBS J. 2016; 283:2823–2835.
Article62. Huang CF, Chang YJ, Hsueh YY, Huang CW, Wang DH, Huang TC, Wu YT, Su FC, Hughes M, Chuong CM, Wu CC. Assembling composite dermal papilla spheres with adipose-derived stem cells to enhance hair follicle induction. Sci Rep. 2016; 6:26436.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of a Hair Tonic Containing Human Umbilical Cord Blood Mesenchymal Stem Cell-derived Conditioned Media in Patients with Androgenetic Alopecia
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Hair follicle stem cells and mitochondria