Cancer Res Treat.
2018 Jul;50(3):926-935. 10.4143/crt.2017.296.
Clinical Risk Factors Influencing Dental Developmental Disturbances in Childhood Cancer Survivors
- Affiliations
-
- 1Department of Pediatric Dentistry, Yonsei University College of Dentistry, Seoul, Korea.
- 2Department of Pharmacology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea. JWHAN@yuhs.ac
- 4Department of Pediatric Hematology-Oncology, Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea.
- 5Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2417881
- DOI: http://doi.org/10.4143/crt.2017.296
Abstract
- PURPOSE
Although studies regarding dental developmental disturbances after childhood cancer treatment have increased, they have many limitations. Studies analyzing the significance of independent clinical risk factors with regard to the dental health status are also rare. We aimed to investigate the risk factors for dental developmental disturbances, particularly severe disturbances, in childhood cancer survivors (CCS).
MATERIALS AND METHODS
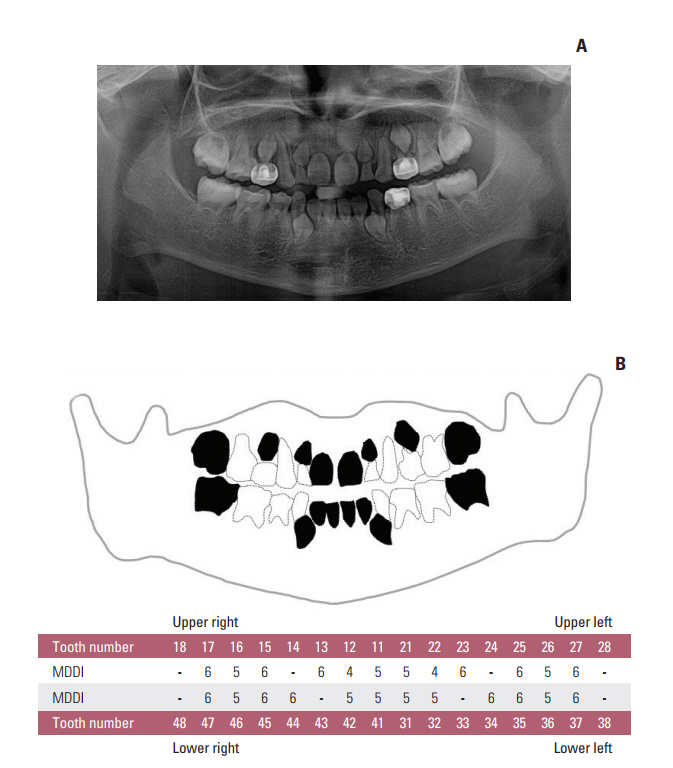
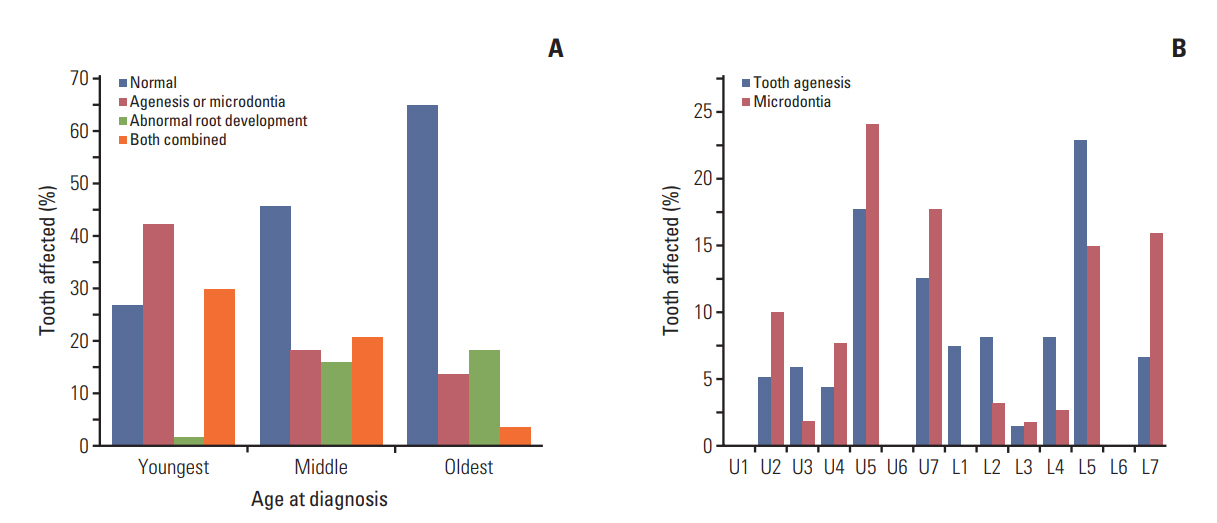
Oral examinations and retrospective reviews of medical and panoramic radiographs were performed for 196 CCS (mean age, 15.6 years). Cancer type, age at diagnosis, treatment modality, type and accumulated dose of administered drugs, and dose and site of radiation were recorded. Dental developmental disturbances were diagnosed using panoramic radiographs and graded for severity according to the Modified Dental Defect Index (MDDI). Descriptive statistics and multivariate analyseswere performed to determine the association between dental abnormalities and clinical factors.
RESULTS
In total, 109 CCS (55.6%) exhibited at least one dental anomaly, and the median value of MDDI was 2.5. Microdontia (30.6%) was the most prevalent anomaly, followed by tooth agenesis (20.4%), V-shaped roots (14.8%), and taurodontism (10.2%). Multivariate analysis revealed that a young age at diagnosis (≤ 3 years), a history of hematopoietic stem cell transplantation, the use of multiple classes of chemotherapeutic agents (≥ 4 classes), and the use of heavy metal agents were significant risk factors for severe dental disturbances.
CONCLUSION
CCS with any of the above risk factors for severe developmental disturbances should be comprehensively followed up to minimize adverse consequences to their dental development and preserve their future dental health.
MeSH Terms
Figure
Cited by 1 articles
-
The Broad Variability in Dental Age Observed among Childhood Survivors Is Cancer Specific
Patrycja Proc, Joanna Szczepańska, Małgorzata Zubowska, Beata Zalewska-Szewczyk, Wojciech Młynarski
Cancer Res Treat. 2021;53(1):252-260. doi: 10.4143/crt.2020.275.
Reference
-
References
1. Kaste SC, Hopkins KP, Jones D, Crom D, Greenwald CA, Santana VM. Dental abnormalities in children treated for acute lymphoblastic leukemia. Leukemia. 1997; 11:792–6.
Article2. Avsar A, Elli M, Darka O, Pinarli G. Long-term effects of chemotherapy on caries formation, dental development, and salivary factors in childhood cancer survivors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:781–9.3. Carrillo CM, Correa FN, Lopes NN, Fava M, Odone Filho V. Dental anomalies in children submitted to antineoplastic therapy. Clinics (Sao Paulo). 2014; 69:433–7.
Article4. Sonis AL, Tarbell N, Valachovic RW, Gelber R, Schwenn M, Sallan S. Dentofacial development in long-term survivors of acute lymphoblastic leukemia: a comparison of three treatment modalities. Cancer. 1990; 66:2645–52.
Article5. Gawade PL, Hudson MM, Kaste SC, Neglia JP, Constine LS, Robison LL, et al. A systematic review of dental late effects in survivors of childhood cancer. Pediatr Blood Cancer. 2014; 61:407–16.
Article6. Proc P, Szczepanska J, Skiba A, Zubowska M, Fendler W, Mlynarski W. Dental anomalies as late adverse effect among young children treated for cancer. Cancer Res Treat. 2016; 48:658–67.
Article7. Holtta P, Alaluusua S, Saarinen-Pihkala UM, Wolf J, Nystrom M, Hovi L. Long-term adverse effects on dentition in children with poor-risk neuroblastoma treated with high-dose chemotherapy and autologous stem cell transplantation with or without total body irradiation. Bone Marrow Transplant. 2002; 29:121–7.
Article8. Oguz A, Cetiner S, Karadeniz C, Alpaslan G, Alpaslan C, Pinarli G. Long-term effects of chemotherapy on orodental structures in children with non-Hodgkin's lymphoma. Eur J Oral Sci. 2004; 112:8–11.
Article9. Duggal MS. Root surface areas in long-term survivors of childhood cancer. Oral Oncol. 2003; 39:178–83.
Article10. Dahllof G, Rozell B, Forsberg CM, Borgstrom B. Histologic changes in dental morphology induced by high dose chemotherapy and total body irradiation. Oral Surg Oral Med Oral Pathol. 1994; 77:56–60.11. Lyaruu DM, van Duin MA, Bervoets TJ, Bronckers AL, Woltgens JH. Daunorubicin-induced pathology in the developing hamster molar tooth germ in vitro. Cancer Detect Prev. 1999; 23:343–50.
Article12. Weidmann AG, Komor AC, Barton JK. Targeted chemotherapy with metal complexes. Comments Mod Chem A Comments Inorg Chem. 2014; 34:114–23.
Article13. Satoh H, Uesugi Y, Kawabata T, Mori K, Fujii F, Kashimoto Y, et al. Morphological classification of dental lesions induced by various antitumor drugs in mice. Toxicol Pathol. 2001; 29:292–9.
Article14. Seifrtova M, Havelek R, Cmielova J, Jiroutova A, Soukup T, Bruckova L, et al. The response of human ectomesenchymal dental pulp stem cells to cisplatin treatment. Int Endod J. 2012; 45:401–12.15. Holtta P, Alaluusua S, Saarinen-Pihkala UM, Peltola J, Hovi L. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer. 2005; 103:181–90.
Article16. Goho C. Chemoradiation therapy: effect on dental development. Pediatr Dent. 1993; 15:6–12.17. Dury DC, Roberts MW, Miser JS, Folio J. Dental root agenesis secondary to irradiation therapy in a case of rhabdomyosarcoma of the middle ear. Oral Surg Oral Med Oral Pathol. 1984; 57:595–9.
Article18. Nasman M, Forsberg CM, Dahllof G. Long-term dental development in children after treatment for malignant disease. Eur J Orthod. 1997; 19:151–9.
Article19. Ooshima T, Ishida R, Mishima K, Sobue S. The prevalence of developmental anomalies of teeth and their association with tooth size in the primary and permanent dentitions of 1650 Japanese children. Int J Paediatr Dent. 1996; 6:87–94.
Article20. Chung CJ, Han JH, Kim KH. The pattern and prevalence of hypodontia in Koreans. Oral Dis. 2008; 14:620–5.
Article21. Ezoddini AF, Sheikhha MH, Ahmadi H. Prevalence of dental developmental anomalies: a radiographic study. Community Dent Health. 2007; 24:140–4.22. Guideline on dental management of pediatric patients receiving chemotherapy, hematopoietic cell transplantation, and/or radiation therapy. Pediatr Dent. 2016; 38:334–42.23. Dirican B, Ozen J, Beyzadeoglu M, Oysul K, Surenkok S, Sipahi C. In vitro evaluation of head and neck radiation shields used to reduce exit dose. Int J Prosthodont. 2006; 19:462–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of Risk Factors for Dental Developmental Disorders in Pediatric Cancer Survivors
- Factors Affecting Social Adjustment of Childhood Cancer Survivors
- A Path Analysis on Factors Influencing Second Primary Cancer Screening Practices in Stomach, Colon, and Breast Cancer Survivors
- Long-term follow-up study and long-term care of childhood cancer survivors
- Bone mineral density deficits in childhood cancer survivors: Pathophysiology, prevalence, screening, and management