Cancer Res Treat.
2018 Jul;50(3):872-882. 10.4143/crt.2017.283.
NUDT15 Variants Cause Hematopoietic Toxicity with Low 6-TGN Levels in Children with Acute Lymphoblastic Leukemia
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hhkoo@skku.edu
- 2Department of Pediatrics, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Chung-Ang University Hospital, Seoul, Korea.
- 4Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. suddenbz@skku.edu
- 5Department of Pediatrics, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- KMID: 2417876
- DOI: http://doi.org/10.4143/crt.2017.283
Abstract
- PURPOSE
We aimed to identify the impact of NUDT15 variants on thiopurine intolerance and 6-thioguanine nucleotide (6-TGN) levels in Korean children with acute lymphoblastic leukemia (ALL).
MATERIALS AND METHODS
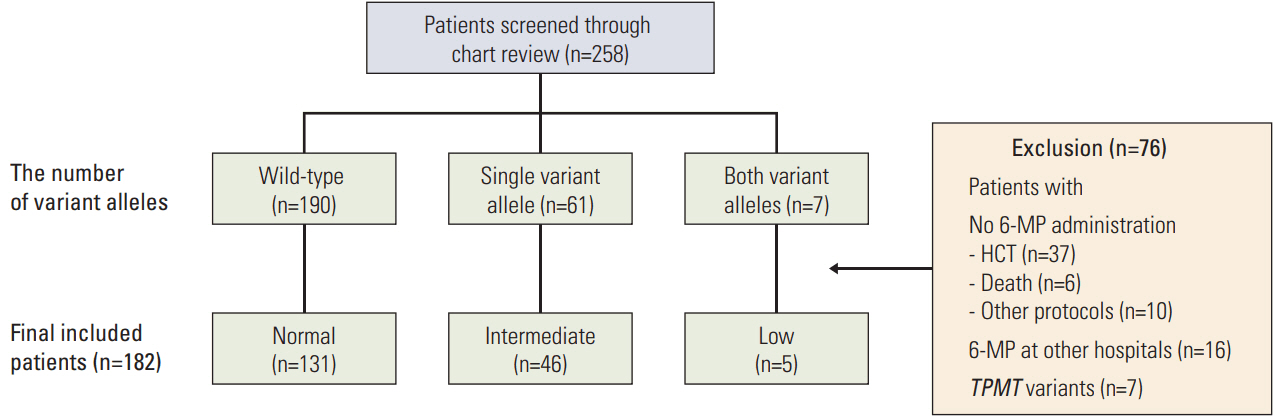
Genotyping of NUDT15 was tested in 258 patients with ALL registered at Samsung Medical Center. Patients were classified into normal-activity (wild-type), intermediate-activity (heterozygous variant), and low-activity groups (homozygous or compound heterozygous variant). Clinical and laboratory features during the first year of maintenance therapy were investigated.
RESULTS
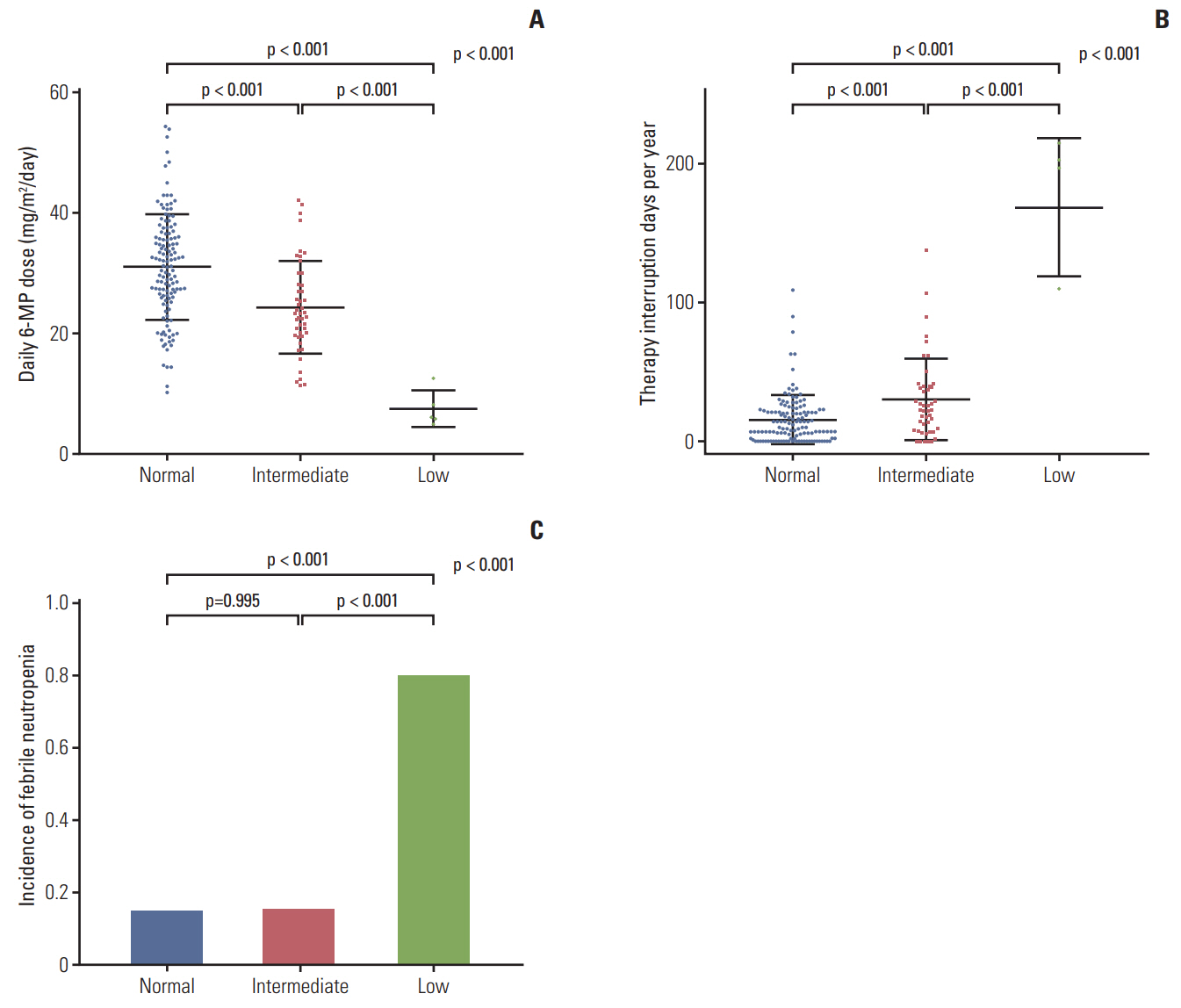
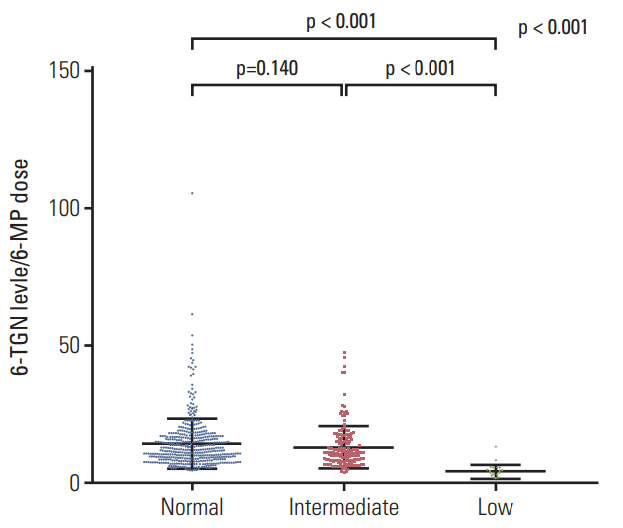
A total of 182 patients were included in the final analysis. There were five (2.7%), 46 (25.3%), and 131 (72.0%) patients in low-, intermediate-, and normal-activity groups, respectively. The lowest 6-mercaptopurine (6-MP) dose (mg/m2/day) was administered to the low-activity group (low-activity group 7.5 vs. intermediate-activity group 24.4 vs. normalactivity group 31.1, p < 0.01) from three months to a year after beginning maintenance therapy. The low-activity group experienced the longest duration of therapy interruption during the first year (low-activity group 169 days vs. intermediate-activity group 30 days vs. normal-activity group 16 days, p < 0.01). They also showed the lowest blood cell counts and had a longer duration of leukopenia (low-activity group 131 days vs. intermediate-activity group 92 days vs. normal-activity group 59 days, p < 0.01). 6-TGN level and its ratio to 6-MP dose were lowest in the low-activity group.
CONCLUSION
NUDT15 variants cause hematopoietic toxicity with low 6-TGN levels. NUDT15 genotyping should be conducted before administering thiopurine, and dose adjustments require caution regardless of 6-TGN levels.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
NUDT15 Genotyping in Thiopurine Drug Therapy
Jong Kwon Lee, Rihwa Choi, Soo-Youn Lee
Lab Med Online. 2022;12(4):217-226. doi: 10.47429/lmo.2022.12.4.217.
Reference
-
References
1. Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011; 29:551–65.
Article2. Lilleyman JS, Lennard L. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet. 1994; 343:1188–90.
Article3. Childhood ALL Collaborative Group. Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12 000 randomised children. Lancet. 1996; 347:1783–8.4. Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013; 14:199–209.
Article5. Fotoohi AK, Coulthard SA, Albertioni F. Thiopurines: factors influencing toxicity and response. Biochem Pharmacol. 2010; 79:1211–20.
Article6. Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J. Mercaptopurine/Methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol. 2014; 36:503–17.7. Bhatia S, Landier W, Hageman L, Kim H, Chen Y, Crews KR, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2014; 124:2345–53.
Article8. Takatsu N, Matsui T, Murakami Y, Ishihara H, Hisabe T, Nagahama T, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009; 24:1258–64.
Article9. Koren G, Ferrazini G, Sulh H, Langevin AM, Kapelushnik J, Klein J, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990; 323:17–21.
Article10. Relling MV, Hancock ML, Boyett JM, Pui CH, Evans WE. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999; 93:2817–23.
Article11. Bhatia S, Landier W, Hageman L, Chen Y, Kim H, Sun CL, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: a Children's Oncology Group Study. JAMA Oncol. 2015; 1:287–95.12. Lennard L, Lilleyman JS, Van Loon J, Weinshilboum RM. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990; 336:225–9.
Article13. Evans WE, Hon YY, Bomgaars L, Coutre S, Holdsworth M, Janco R, et al. Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol. 2001; 19:2293–301.
Article14. Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013; 93:324–5.
Article15. Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, et al. Pharmacogenetics: from bench to byte: an update of guidelines. Clin Pharmacol Ther. 2011; 89:662–73.
Article16. Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014; 46:1017–20.
Article17. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015; 33:1235–42.
Article18. Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016; 48:367–73.
Article19. Zgheib NK, Akika R, Mahfouz R, Aridi CA, Ghanem KM, Saab R, et al. NUDT15 and TPMT genetic polymorphisms are related to 6-mercaptopurine intolerance in children treated for acute lymphoblastic leukemia at the Children's Cancer Center of Lebanon. Pediatr Blood Cancer. 2017; 64:146–50.
Article20. Takagi Y, Setoyama D, Ito R, Kamiya H, Yamagata Y, Sekiguchi M. Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison with MTH1 and MTH2. J Biol Chem. 2012; 287:21541–9.21. Kim HT, Choi R, Won HH, Choe YH, Kang B, Lee K, et al. NUDT15 genotype distributions in the Korean population. Pharmacogenet Genomics. 2017; 27:197–200.22. Dervieux T, Meyer G, Barham R, Matsutani M, Barry M, Boulieu R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005; 51:2074–84.
Article23. Lee MN, Kang B, Choi SY, Kim MJ, Woo SY, Kim JW, et al. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015; 21:1054–62.
Article24. Lee MN, Kang B, Choi SY, Kim MJ, Woo SY, Kim JW, et al. Impact of genetic polymorphisms on 6-thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015; 21:2897–908.
Article25. Lennard L, Welch JC, Lilleyman JS. Thiopurine drugs in the treatment of childhood leukaemia: the influence of inherited thiopurine methyltransferase activity on drug metabolism and cytotoxicity. Br J Clin Pharmacol. 1997; 44:455–61.
Article26. Valerie NC, Hagenkort A, Page BD, Masuyer G, Rehling D, Carter M, et al. NUDT15 hydrolyzes 6-thio-deoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 2016; 76:5501–11.27. Ebbesen MS, Nersting J, Jacobsen JH, Frandsen TL, Vetten-ranta K, Abramsson J, et al. Incorporation of 6-thioguanine nucleotides into DNA during maintenance therapy of childhood acute lymphoblastic leukemia-the influence of thiopurine methyltransferase genotypes. J Clin Pharmacol. 2013; 53:670–4.
Article28. Nielsen SN, Grell K, Nersting J, Frandsen TL, Hjalgrim LL, Schmiegelow K. Measures of 6-mercaptopurine and methotrexate maintenance therapy intensity in childhood acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2016; 78:983–94.
Article29. Moriyama T, Nishii R, Lin TN, Kihira K, Toyoda H, Jacob N, et al. The effects of inherited NUDT15 polymorphisms on thiopurine active metabolites in Japanese children with acute lymphoblastic leukemia. Pharmacogenet Genomics. 2017; 27:236–9.
Article30. Kham SK, Soh CK, Liu TC, Chan YH, Ariffin H, Tan PL, et al. Thiopurine S-methyltransferase activity in three major Asian populations: a population-based study in Singapore. Eur J Clin Pharmacol. 2008; 64:373–9.
Article31. Tanaka Y, Kato M, Hasegawa D, Urayama KY, Nakadate H, Kondoh K, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. 2015; 171:109–15.32. Kim H, Seo H, Park Y, Min BJ, Seo ME, Park KD, et al. APEX1 Polymorphism and mercaptopurine-related early onset neutropenia in pediatric acute lymphoblastic leukemia. Cancer Res Treat. 2017. Sep. 4. [Epub]. https://doi.org/10.4143/crt.2017.351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unusual isolated extramedullary relapse of acute lymphoblastic leukemia in the breast despite complete donor hematopoietic chimerism after allogeneic hematopoietic stem cell transplantation
- A Case of Bone Marrow Necrosis Following Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- Immune response induced by live attenuated varicella vaccine(Biken@) in children with acute lymphoblastic leukemia
- Treatment of Acute Lymphoblastic Leukemia in children