J Korean Neurosurg Soc.
2018 Jul;61(4):434-440. 10.3340/jkns.2017.0252.
In Vivo Spinal Distribution of Cy5.5 Fluorescent Dye after Injection via the Lateral Ventricle and Cisterna Magna in Rat Model
- Affiliations
-
- 1Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul, Korea. kmjoo@skku.edu, sobotta72@gmail.com
- 2Single Cell Network Research Center, Sungkyunkwan University School of Medicine, Suwon, Korea.
- 3Stem Cell and Regenerative Medicine Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul, Korea.
- 4Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Medical Education, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Anatomy and Cell Biology, Sungkyunkwan University School of Medicine, Suwon, Korea.
- KMID: 2417318
- DOI: http://doi.org/10.3340/jkns.2017.0252
Abstract
OBJECTIVE
The purpose of this study was to find an optimal delivery route for clinical trials of intrathecal cell therapy for spinal cord injury in preclinical stage.
METHODS
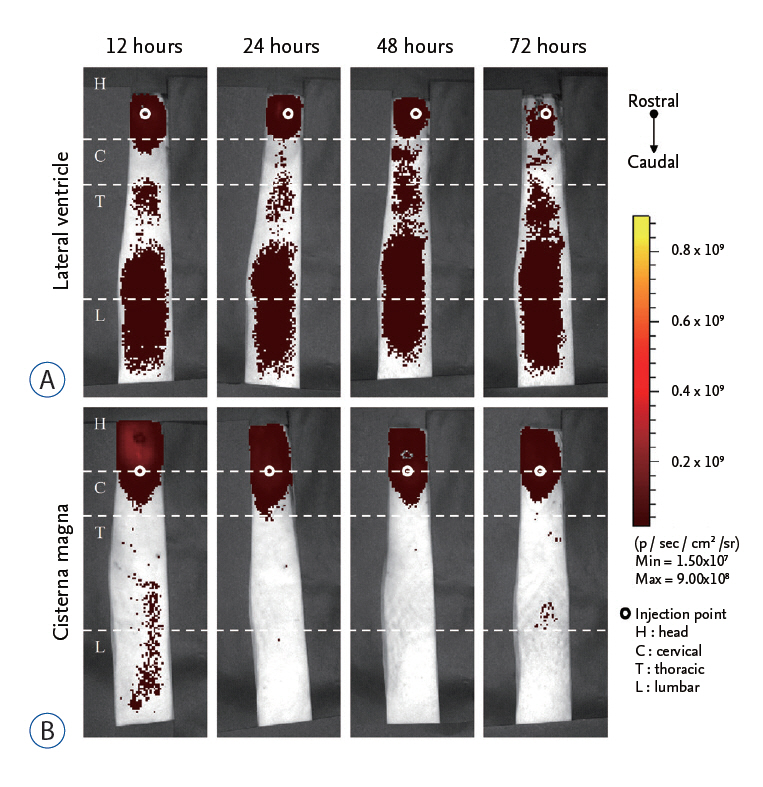
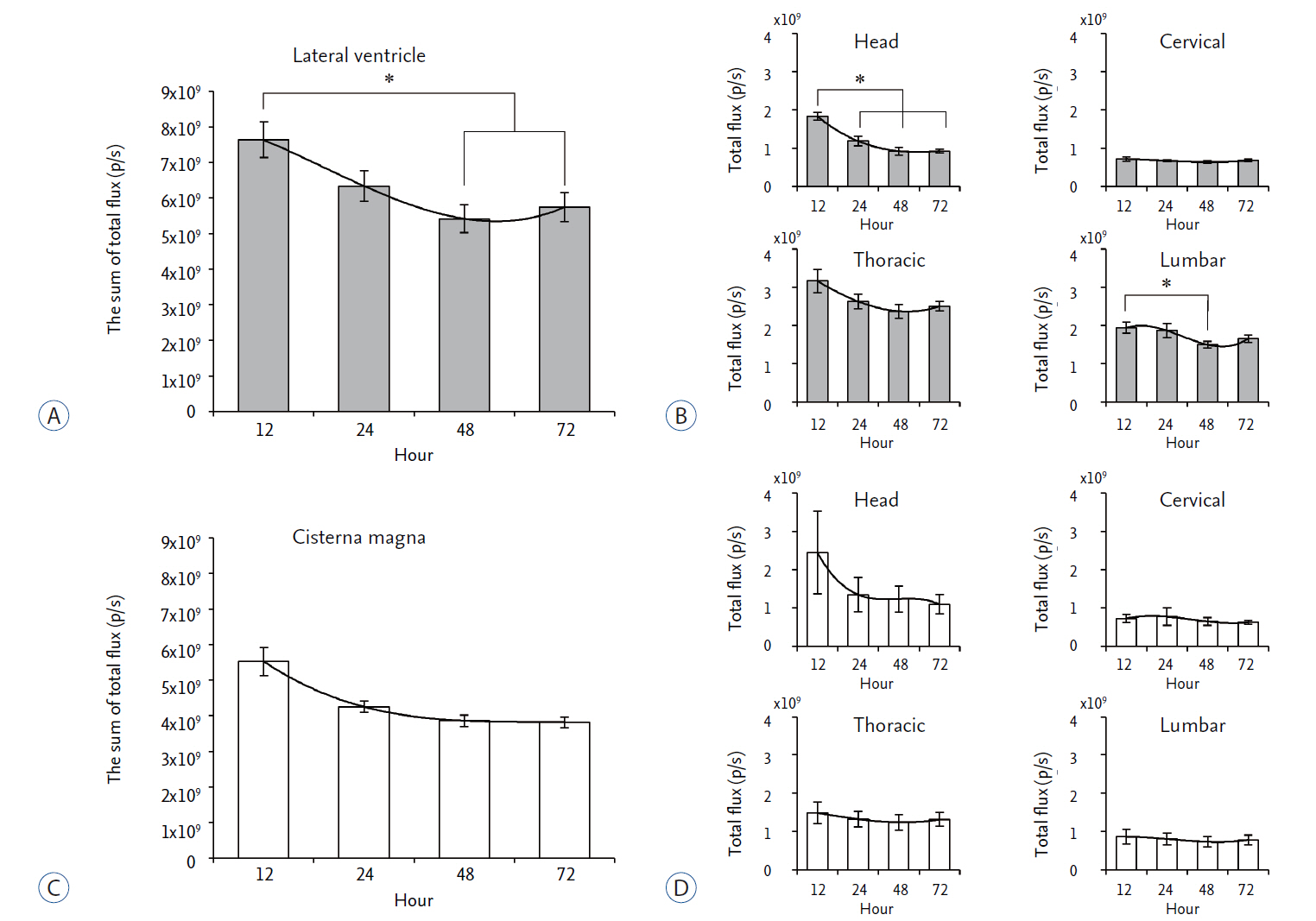
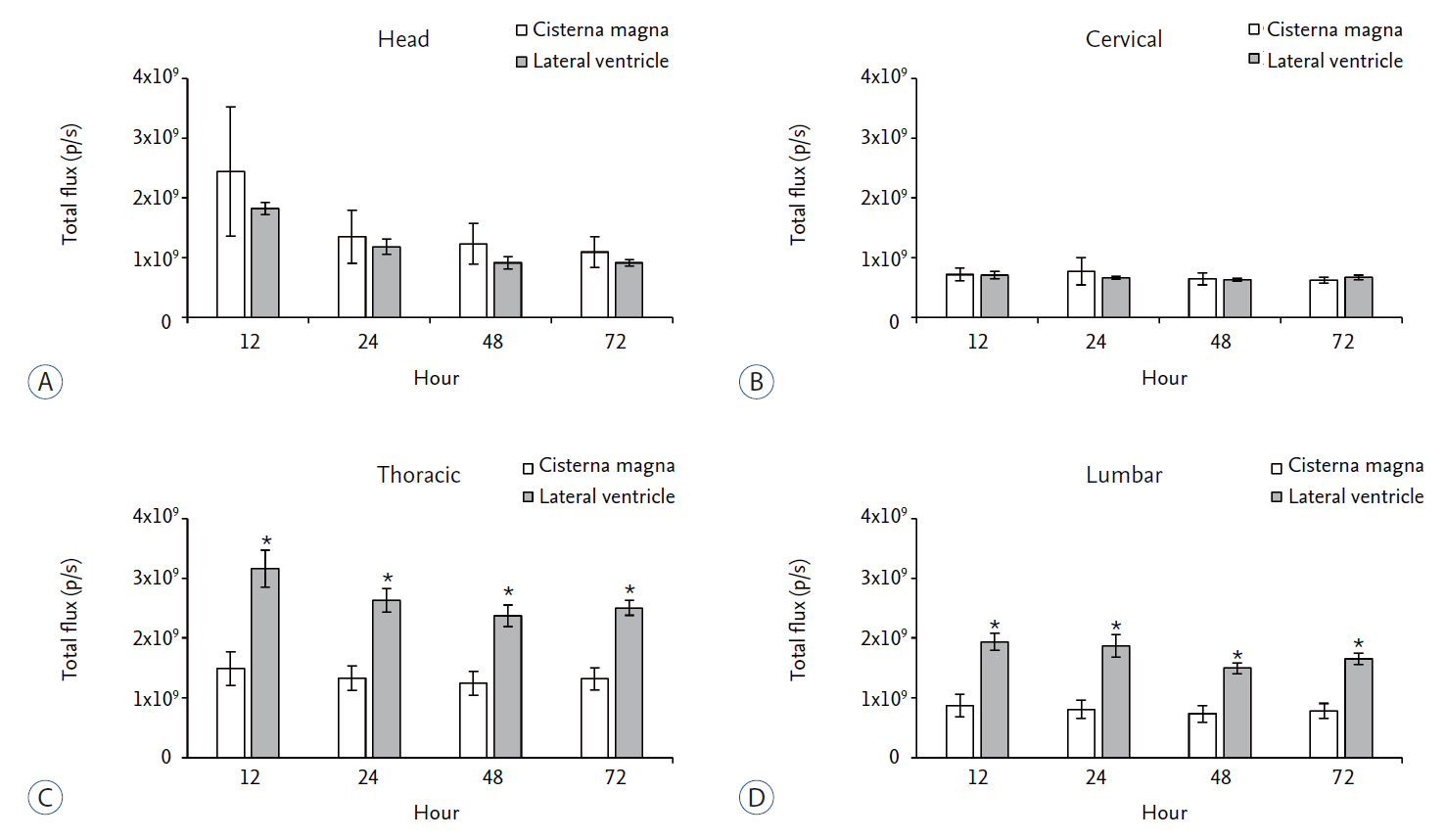
We compared in vivo distribution of Cy5.5 fluorescent dye in the spinal cord region at various time points utilizing in vivo optical imaging techniques, which was injected into the lateral ventricle (LV) or cisterna magna (CM) of rats.
RESULTS
Although CM locates nearer to the spinal cord than the LV, significantly higher signal of Cy5.5 was detected in the thoracic and lumbar spinal cord region at all time points tested when Cy5.5 was injected into the LV. In the LV injection Cy5.5 signal in the thoracic and lumbar spinal cord was observed within 12 hours after injection, which was maintained until 72 hours after injection. In contrast, Cy5.5 signal was concentrated at the injection site in the CM injection at all time points.
CONCLUSION
These data suggested that the LV might be suitable for preclinical injection route of therapeutics targeting the spinal cord to test their treatment efficacy and biosafety for spinal cord diseases in small animal models.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 3:537–544. 2000.
Article2. Boulton M, Flessner M, Armstrong D, Mohamed R, Hay J, Johnston M. Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am J Physiol. 276:R818–R823. 1999.3. Bradbury MW, Lathem W. A flow of cerebrospinal fluid along the central canal of the spinal cord of the rabbit and communications between this canal and the sacral subarachnoid space. J Physiol. 181:785–800. 1965.
Article4. Chen Y, Imai H, Ito A, Saito N. Novel modified method for injection into the cerebrospinal fluid via the cerebellomedullary cistern in mice. Acta Neurobiol Exp (Wars). 73:304–311. 2013.5. Consiglio AR, Lucion AB. Technique for collecting cerebrospinal fluid in the cisterna magna of non-anesthetized rats. Brain Res Brain Res Protoc. 5:109–114. 2000.
Article6. Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 75:363–373. 2014.
Article7. Gao GH, Park MJ, Li Y, Im GH, Kim JH, Kim HN, et al. The use of pH-sensitive positively charged polymeric micelles for protein delivery. Biomaterials. 33:9157–9164. 2012.
Article8. Johnston M, Zakharov A, Koh L, Armstrong D. Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol Appl Neurobiol. 31:632–640. 2005.
Article9. Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 87:2183–2200. 2009.
Article10. Koedel U, Bernatowicz A, Frei K, Fontana A, Pfister HW. Systemically (but not intrathecally) administered IL-10 attenuates pathophysiologic alterations in experimental pneumococcal meningitis. J Immunol. 157:5185–5191. 1996.11. Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 10 Suppl:S42–S50. 2004.
Article12. Liu L, Duff K. technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J Vis Exp. (21):960. 2008.13. Proescholdt MG, Hutto B, Brady LS, Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience. 95:577–592. 2000.
Article14. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 25:E2. 2008.
Article15. Shirao S, Fujisawa H, Kudo A, Kurokawa T, Yoneda H, Kunitsugu I, et al. Inhibitory effects of eicosapentaenoic acid on chronic cerebral vasospasm after subarachnoid hemorrhage: possible involvement of a sphingosylphosphorylcholine-rho-kinase pathway. Cerebrovasc Dis. 26:30–37. 2008.
Article16. van der Flier M, Coenjaerts FE, Mwinzi PN, Rijkers E, Ruyken M, Scharringa J, et al. Antibody neutralization of vascular endothelial growth factor (VEGF) fails to attenuate vascular permeability and brain edema in experimental pneumococcal meningitis. J Neuroimmunol. 160:170–177. 2005.
Article17. Yang HY, Jang MS, Li Y, Lee JH, Lee DS. Multifunctional and redox-responsive self-assembled magnetic nanovectors for protein delivery and dual-modal imaging. ACS Appl Mater Interfaces. 9:19184–19192. 2017.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution and accumulation of Cy5.5-labeled thermally cross-linked superparamagnetic iron oxide nanoparticles in the tissues of ICR mice
- Clinical significance of mega cisterna magna
- The Mega Cisterna Magna: Report of 4 Cases
- Manic Episode Associated with Mega Cisterna Magna
- Studies on Bradycardia by Centrally Administered Atropine in the Rabbit