Nat Prod Sci.
2018 Jun;24(2):82-87. 10.20307/nps.2018.24.2.82.
Determination of Silybin B in the Different Parts of Silybum marianum using HPLC-UV
- Affiliations
-
- 1Department of Integrative Plant Science, Chung-Ang University, Anseong 17546, Korea. slee@cau.ac.kr
- 2Natural Products Research Team, National Marine Biodiversity Institute of Korea, Seocheon 33662, Korea.
- 3Department of Food Science, Gyeongnam National University of Science and Technology, Jinju 52725, Korea.
- 4Imsil Herbal Medicine Association, Imsil 55955, Korea.
- KMID: 2416066
- DOI: http://doi.org/10.20307/nps.2018.24.2.82
Abstract
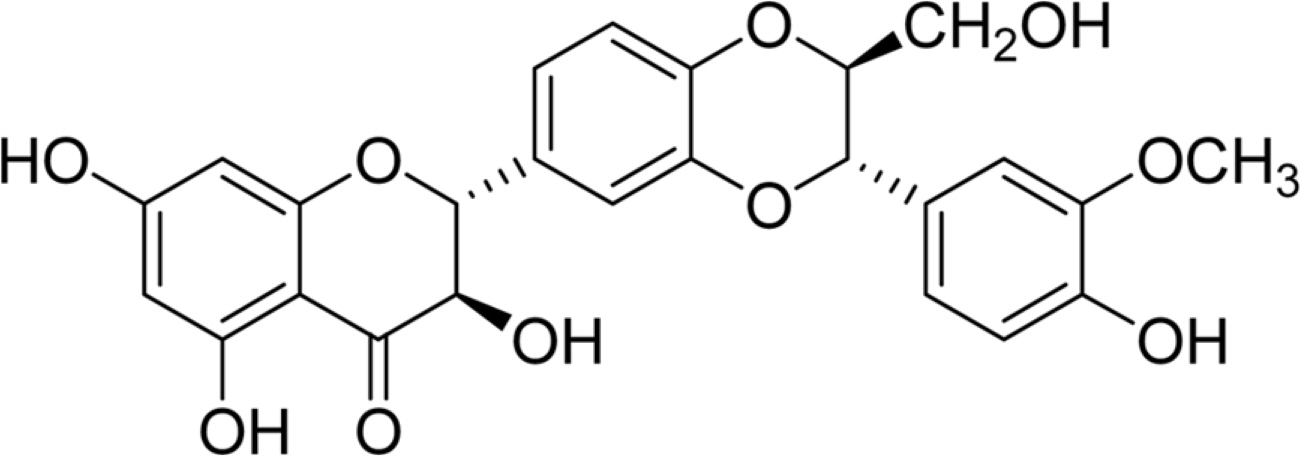
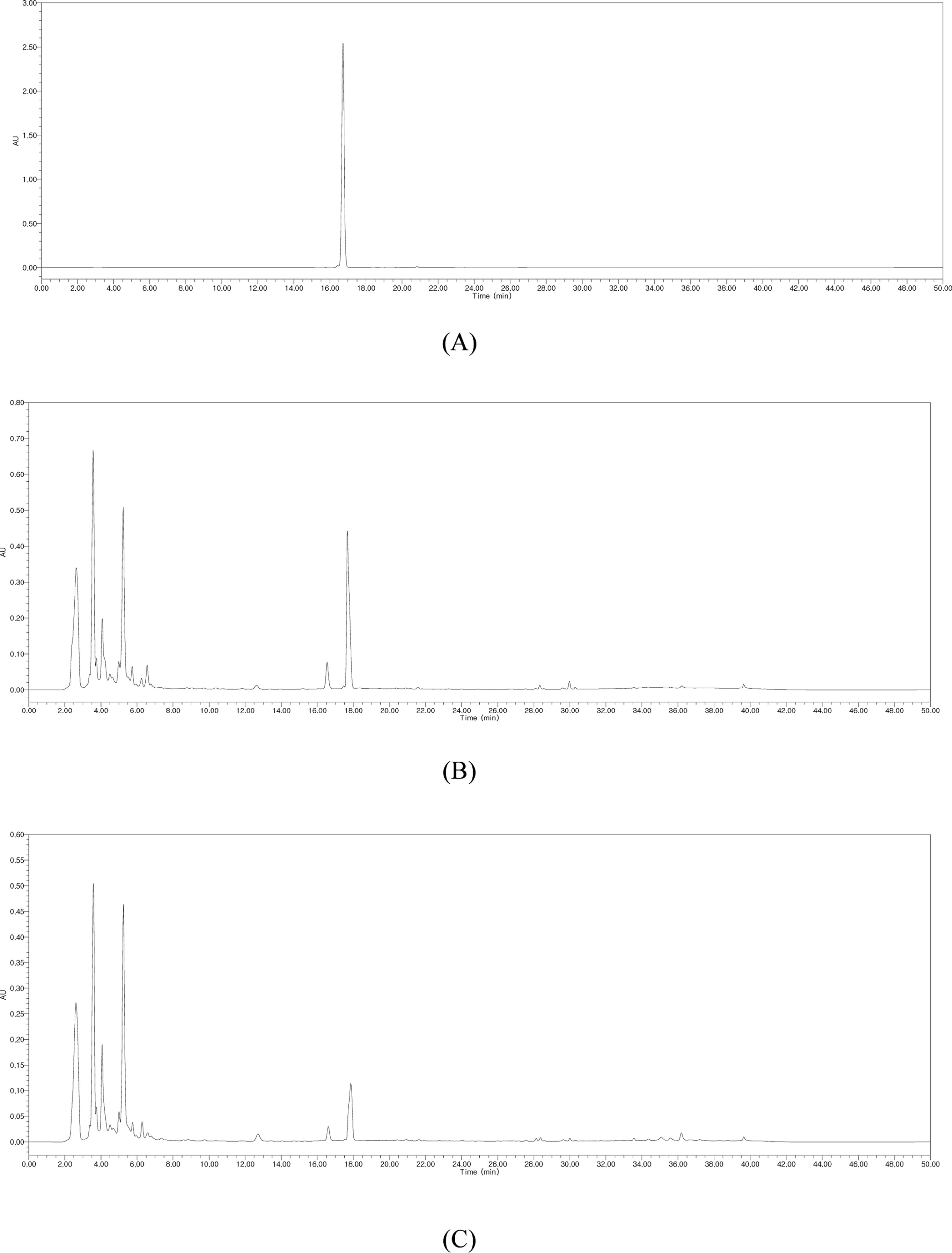
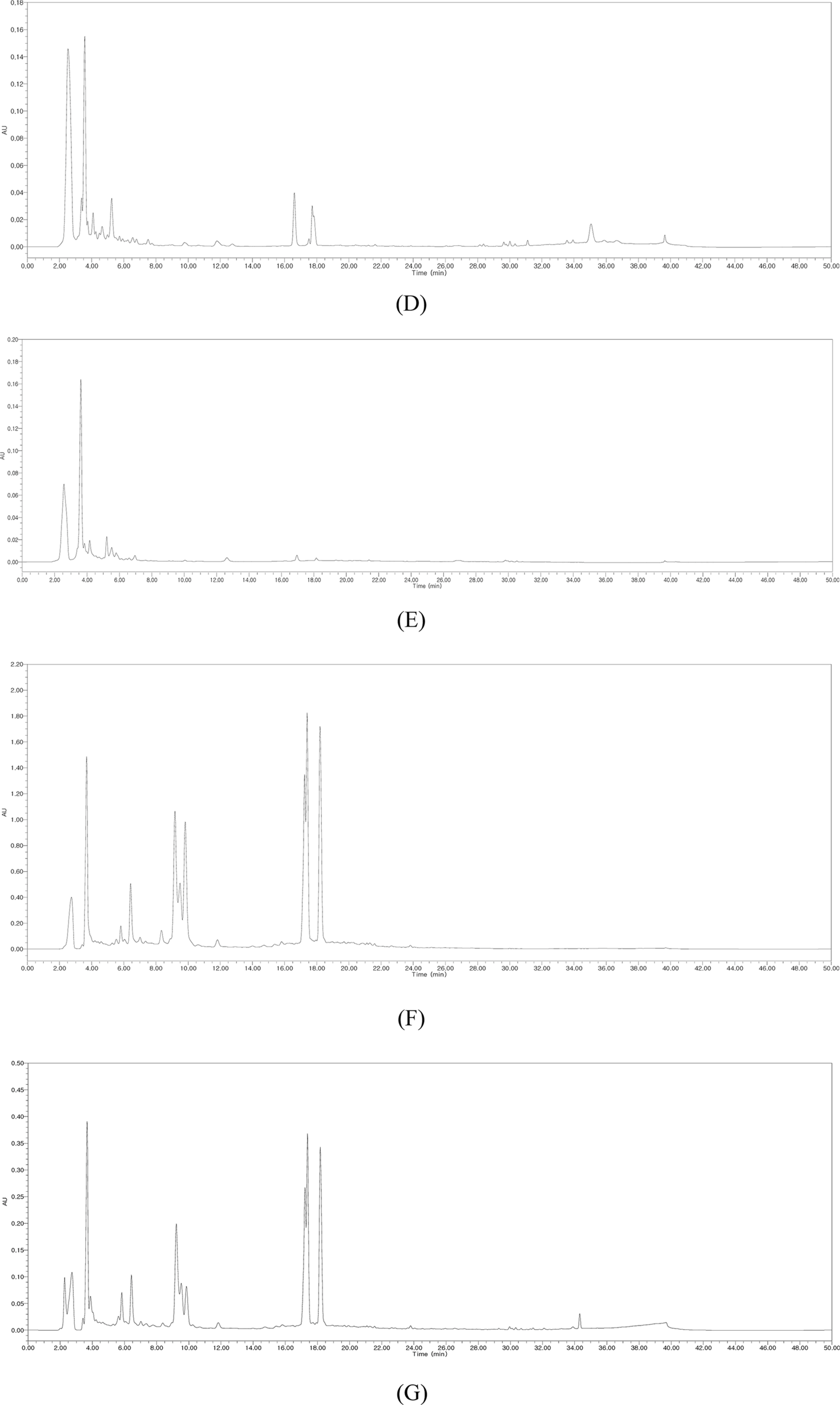
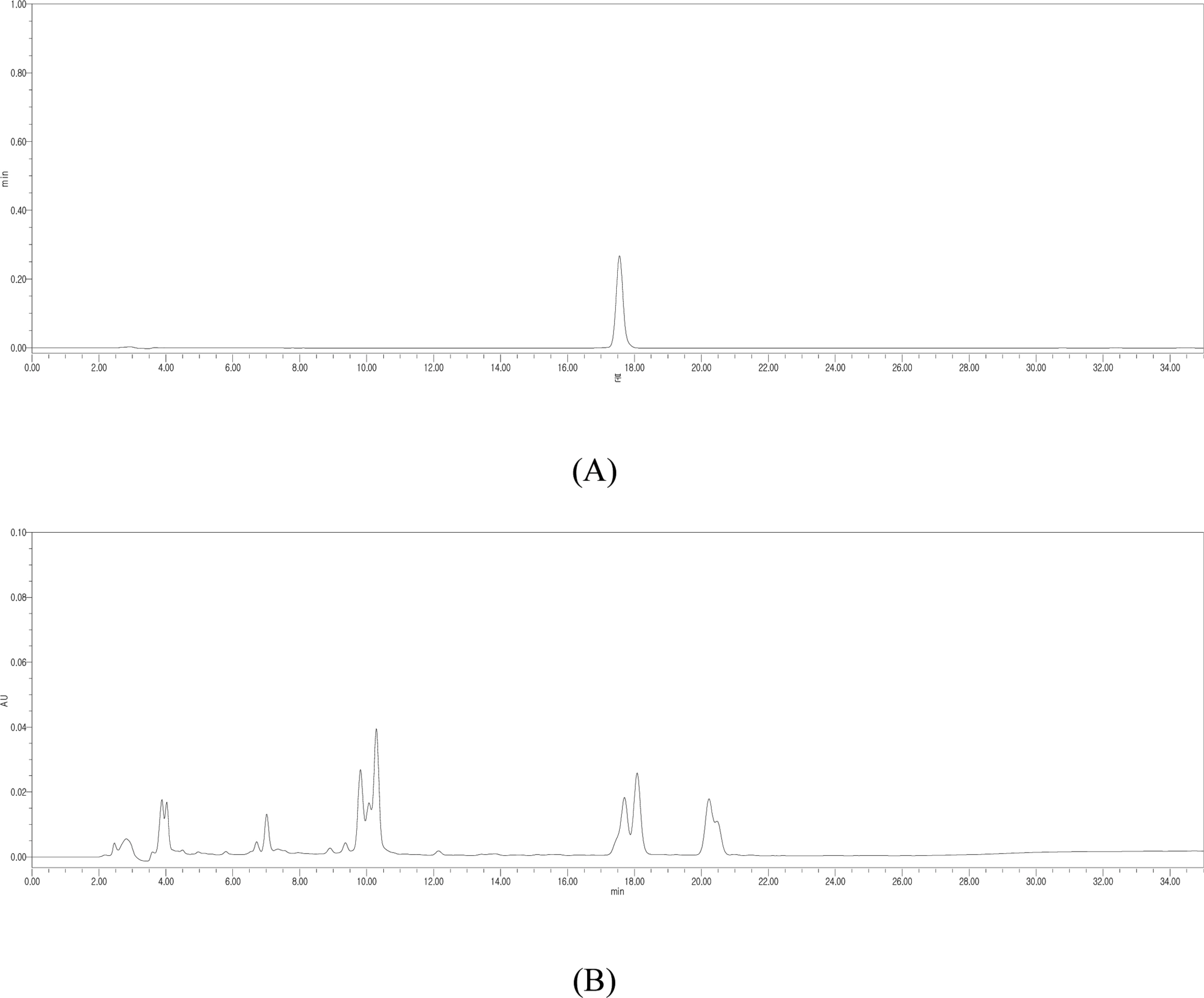
- Silymarin is the standardized extract from Silybum marianum which consists mainly of flavonoids and polyphenols. It is highly regarded for its hepatoprotective ability. Silybin B is a flavonolignan and one of the active components of silymarin. The content of silybin B in various parts of S. marianum was analyzed by HPLC-UV. Results show that the extract of seeds contain the highest amount of silybin B (7.434 mg/g DW). The petioles of S. marianum showed a low content of silybin B. This study revealed that seeds of S. marianum contain high amount of silybin B and could be a good source of the compound.
Keyword
Figure
Reference
-
References
(1). Elwekeel A.., Elfishawy A.., AbouZid S. J.Med. Plants Res. 2013. 7:1665–1669.(2). AbouZid S. F.., Chen S. N.., Pauli G. F.Ind. Crops Prod. 2016. 83:729–737.(3). Abenavoli L.., Capasso R.., Milic N.., Capasso F.Phytother. Res. 2010. 24:1423–1432. . ê.(4). K en V.., Walterová D.Biomed. Pap. Med. Fac. Univ. Palacky r Olomouc Czech Repub. 2005. 149:29–41. . ê ê ê.(5). Kvasni ka F.., Bíba B.., Šev ík R.., Vold ich M.., Krátká J. J.c c Chromatogr. A. 2003. 990:239–245. . ê r Chromatogr. A. 2003 990.(6). Saller R.., Meier R.., Brignoli R.Drugs. 2001. 61:2035–2063.(7). Hwang I. -S.., Han E. -J.., Bak J. -S.., Kim J. -G.., Chough N.J. Nat. Prod. Sci. 2006. 12:166–173.(8). Kim N. C.., Graf T. N.., Sparacino C. M.., Wani M. C.., Wall M. E.Org. Biomol. Chem. 2003. 1:1684–1689.(9). Shaker E.., Mahmoud H.., Mnaa S.Food Chem. Toxicol. 2010. 48:803–806.(10). Tamayo C.., Diamond S.Integr. Cancer Ther. 2007. 6:146–157.(11). Csupor D.., Csorba A.., Hohmann J. J.Pharm. Biomed. Anal. 2016. 130:301–317.(12). Loguercio C.., Festi D.World J. Gastroenterol. 2011. 17:2288–2301.(13). Trouillas P.., Marsal P.., Svobodová A.., Vostálová J.., Gažák R.., ê Hrbác J.., Sedmera P.., K en V.., Lazzaroni R.., Duroux J. L.., Walterová r D. J.Phys. Chem. A. 2008. 112:1054–1063.(14). Trappoliere M.., Caligiuri A.., Schmid M.., Bertolani C.., Failli P.., Vizzutti F.., Novo E.., di Manzano C.., Marra F.., Loguercio C.., Pinzani M. J.Hepatol. 2009. 50:1102–1111.(15). Scambia G.., De Vincenzo R.., Ranelletti P. O.., Panici P. B.., Ferrandina G.., D'Agostino G.., Fattorossi A.., Bombardelli E.., Mancuso S.Eur. J. Cancer. 1996. 32:877–882.(16). Tyagi A.., Bhatia N.., Condon M. S.., Bosland M. C.., Agarwal C.., Agarwal R.Prostate. 2002. 53:211–217.(17). Zheng D.., Wang Y.., Zhang D.., Liu Z.., Duan C.., Jia L.., Wang F.., Liu Y.., Liu G.., Hao L.., Zhang Q.Cancer Lett. 2011. 307:158–164.(18). Lee D. G.., Kim H. K.., Park Y.., Park S. C.., Woo E. R.., Jeong H. G.., Hahm K. S.Arch. Pharm. Res. 2003. 26:597–600.(19). Cappelletti E. M.., Caniato R.Herba Hung. 1984. 23:53–66.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Pharmacological Properties of Silymarin and Its Constituents
- Protective effect of the aerial parts of Silybum marianum against amyloid β protein (25-35)-induced neuronal death in cultured neurons
- Quantitative Determination of Bakkenolide D in Petasites japonicus and Farfugium japonicum by HPLC/UV
- Simultaneous Determination of Four Compounds from Cercidiphyllum japonicum Using HPLC-UV Analysis
- Validation of High Performance Liquid Chromatographic Method with UV Detector for the Determination of Di(2-ethylhexyl)Phthalate in Plasma in some Korean Male Workers