Blood Res.
2018 Mar;53(1):41-48. 10.5045/br.2018.53.1.41.
Global hemostatic assay of different target procoagulant activities of factor VIII and factor IX
- Affiliations
-
- 1Korea Hemophilia Foundation, Seoul, Korea. gowho@hanmail.net
- 2Mogam Institute for Biomedical Research, Yongin, Korea.
- KMID: 2414363
- DOI: http://doi.org/10.5045/br.2018.53.1.41
Abstract
- BACKGROUND
Korean National Health Insurance reimburses factor VIII (FVIII) and factor IX (FIX) clotting factor concentrate (CFC) infusions to discrepant activity levels, allowing elevation of FVIII activity to 60 IU/dL and FIX to 40 IU/dL. We aimed to assess hemostatic response to these target levels using global hemostatic assays.
METHODS
We enrolled 34 normal healthy men, 34 patients with hemophilia A, and 36 with hemophilia B, with residual factor activity of 3 IU/dL or less and without inhibitors. Patients with hemophilia A and B received injected CFCs according to reimbursement guidelines. Fifteen minutes after injection, we assessed hemostatic response with global hemostatic assays: thrombin generation assay (TGA), thromboelastography (TEG), and clot waveform analysis (CWA).
RESULTS
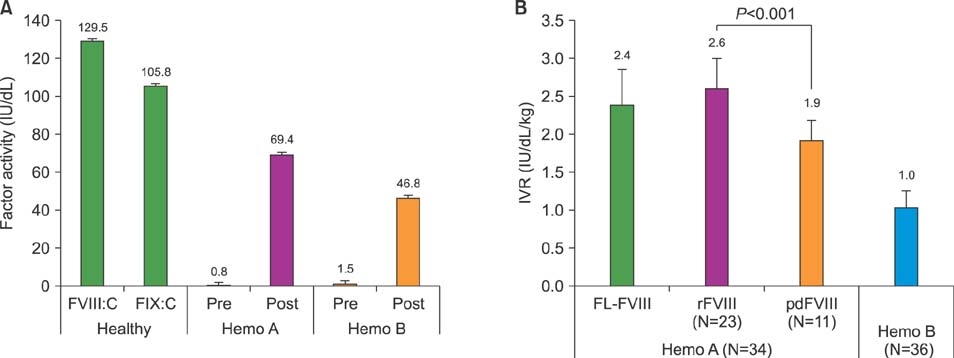
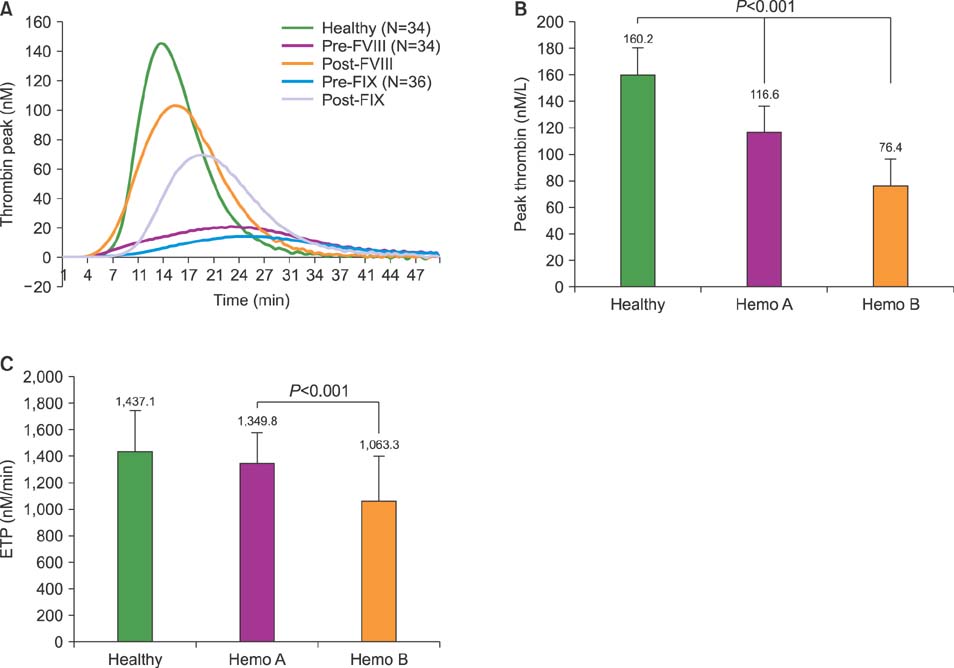
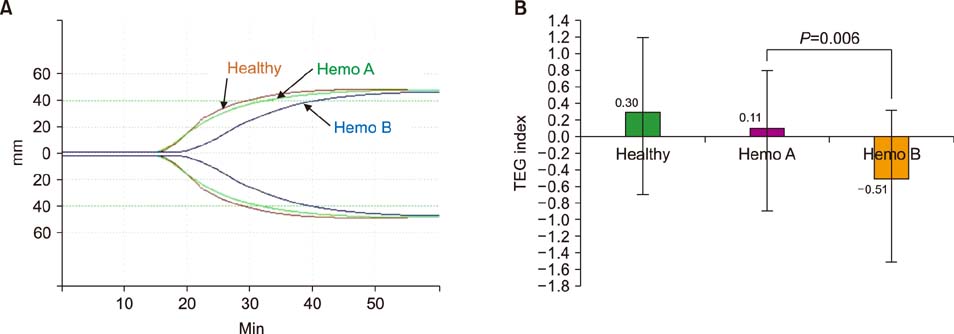
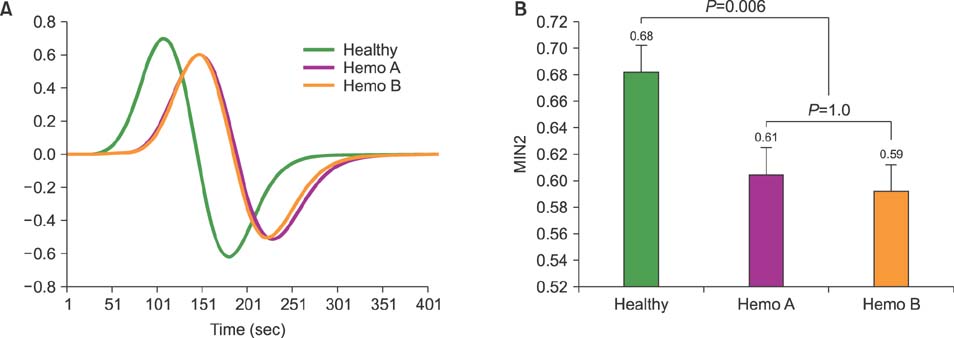
Normal healthy men and patients with hemophilia A and B were 36.7, 37.2, and 35.1 years old, respectively. FVIII and recombinant FIX concentrate doses were 28.8 IU/kg and 43.6 IU/kg. Post-infusion FVIII activity rose from 0.5 IU/dL to 69.4 IU/dL, while FIX activity rose from 1.4 IU/dL to 46.8 IU/dL. Post-infusion peak thrombin concentrations in hemophilia A and B were 116.6 nM/L and 76.4 nM/L (P < 0.001). Post-infusion endogenous thrombin potential (ETP) in hemophilia A and B was 1349.8 nM/min and 915.6 nM (P < 0.001). TEG index of hemophilia A and B was 0.11 and −0.51 (P=0.006).
CONCLUSION
Current reimbursed doses for FIX concentrates are insufficient to achieve hemostatic responses comparable to those after reimbursed doses for FVIII concentrates in terms of peak thrombin concentration, ETP, and TEG index.
Keyword
MeSH Terms
Figure
Reference
-
1. White GC 2nd, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001; 85:560.2. Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013; 19:e1–e47.
Article3. Escobar MA. Products used to treat hemophilia: dosing. In : Lee CA, Berntorp EE, Hoots WK, editors. Textbook of hemophilia. 3rd ed. Oxford, UK: Wiley-Blackwell;2014. p. 180–184.4. Lozier JN, Kessler CM. Clinical aspects and therapy of hemophilia. In : Hoffman R, Benz EJ, Shattil SJ, editors. Hematology: basic principles and practice. 4th ed. Philadelphia, PA: Elsevier;2005. p. 2047–2069.5. Scott JP, Flood VH. Hereditary clotting factor deficiencies (bleeding disorders). In : Kliegman RM, Stanton BF, Geme JW, Schor NF, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia, PA: Elsevier;2016. p. 2384–2389.6. Makris M. Is VIII worse than IX. Blood. 2009; 114:750–751.
Article7. Lowe GD, Ludlam CA. Less severe bleeding in hemophilia B than in hemophilia A. J Thromb Haemost. 2008; 6:1982–1983.
Article8. den Uijl IE, Roosendaal G, Fischer K. Insufficient evidence to suggest less stringent therapy in hemophilia B? Blood. 2009; 114:4907.
Article9. Tagariello G, Iorio A, Santagostino E, et al. Comparison of the rates of joint arthroplasty in patients with severe factor VIII and IX deficiency: an index of different clinical severity of the 2 coagulation disorders. Blood. 2009; 114:779–784.
Article10. Santagostino E, Mancuso ME, Tripodi A, et al. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost. 2010; 8:737–743.
Article11. Chang P, Aronson DL, Borenstein DG, Kessler CM. Coagulant proteins and thrombin generation in synovial fluid: a model for extravascular coagulation. Am J Hematol. 1995; 50:79–83.
Article12. Clausen N, Petrini P, Claeyssens-Donadel S, et al. Similar bleeding phenotype in young children with haemophilia A or B: a cohort study. Haemophilia. 2014; 20:747–755.
Article13. van Hylckama Vlieg A, van der Linden IK, Bertina RM, Rosendaal FR. High levels of factor IX increase the risk of venous thrombosis. Blood. 2000; 95:3678–3682.
Article14. Den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand. Haemophilia. 2011; 17:849–853.
Article15. Turecek PL, Abbühl B, Tangada SD, et al. Nonacog gamma, a novel recombinant factor IX with low factor IXa content for treatment and prophylaxis of bleeding episodes. Expert Rev Clin Pharmacol. 2015; 8:163–177.
Article16. Kim DH, Ko SH, Kim DC, Lee SK, Song HS. The effects of measurement time and blood temperature on thromboelastographic parameters. Korean J Anesthesiol. 2002; 42:306–311.
Article17. Beltrán-Miranda CP, Khan A, Jaloma-Cruz AR, Laffan MA. Thrombin generation and phenotypic correlation in haemophilia A. Haemophilia. 2005; 11:326–334.
Article18. Chitlur M. Challenges in the laboratory analyses of bleeding disorders. Thromb Res. 2012; 130:1–6.
Article19. Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003; 33:4–15.
Article20. Feng D, Stafford KA, Broze GJ, Stafford DW. Evidence of clinically significant extravascular stores of factor IX. J Thromb Haemost. 2013; 11:2176–2178.
Article21. Osterud B, Rapaport SI. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977; 74:5260–5264.
Article22. Cooley B, Funkhouser W, Monroe D, et al. Prophylactic efficacy of BeneFIX vs Alprolix in hemophilia B mice. Blood. 2016; 128:286–292.
Article23. Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007; 109:566–576.
Article24. Hoffman M, Monroe DM 3rd. A cell-based model of hemostasis. Thromb Haemost. 2001; 85:958–965.
Article25. Lawson JH, Mann KG. Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. J Biol Chem. 1991; 266:11317–11327.
Article26. Crawley JT, Zanardelli S, Chion CK, Lane DA. The central role of thrombin in hemostasis. J Thromb Haemost. 2007; 5:Suppl 1. 95–101.
Article27. Biggs R, Macfarlane RG. Haemophilia and related conditions: a survey of 187 cases. Br J Haematol. 1958; 4:1–27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent Cerebral Venous Thrombosis Associated with Elevated Factor VIII
- Recent Advance of Pharmacotherapy in Hemophilia
- The activity of factor VIII and IX of cord blood at mid-trimester in fetuses without hemophilia
- Iliacus Hematoma with Femoral Neuropathy in Hemophilia: A Case report
- Factor VIII inhibitors in Korean hemophiliacs-I. prevalence of factor VIII inhibitors