J Vet Sci.
2016 Sep;17(3):307-314. 10.4142/jvs.2016.17.3.307.
Development and evaluation of a competitive enzyme-linked immunosorbent assay using a monoclonal antibody for diagnosis of severe fever with thrombocytopenia syndrome virus in bovine sera
- Affiliations
-
- 1Division of Viral Disease, Animal and Plant Quarantine Agency, Anyang 14086, Korea. shinyk2009@korea.kr
- 2Division of Foreign Animal Disease, Animal and Plant Quarantine Agency, Anyang 14086, Korea.
- KMID: 2413129
- DOI: http://doi.org/10.4142/jvs.2016.17.3.307
Abstract
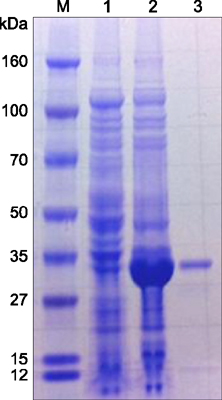
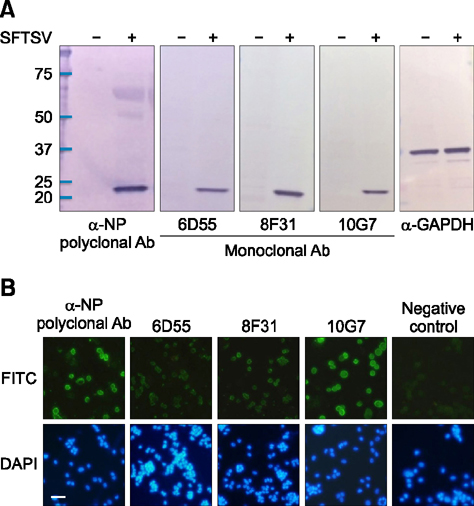
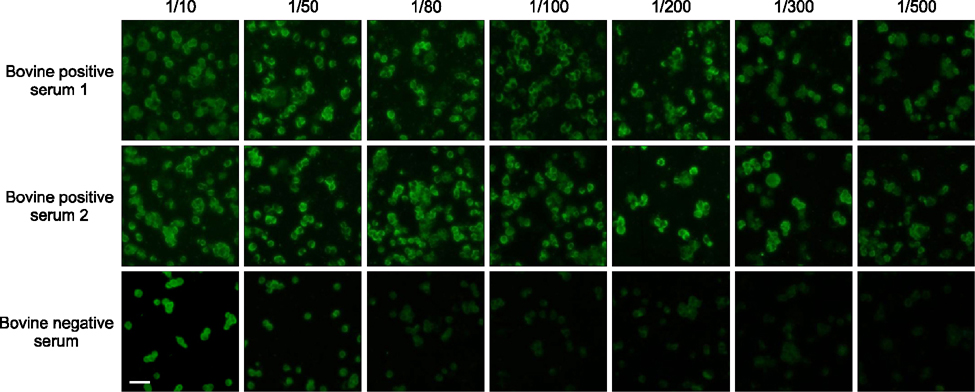
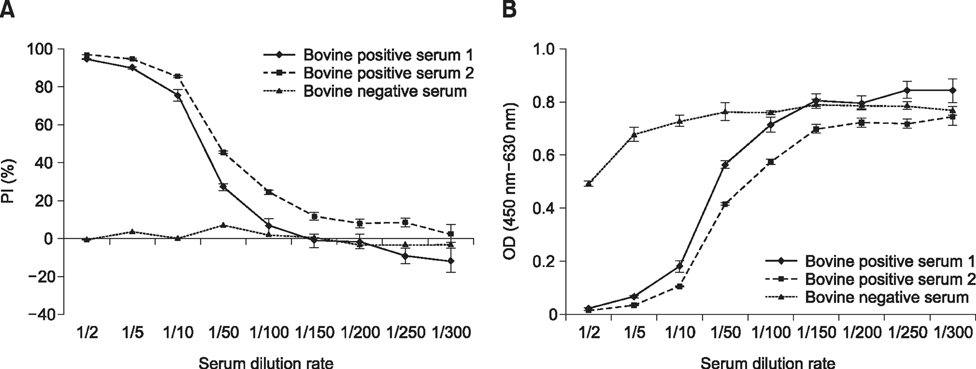
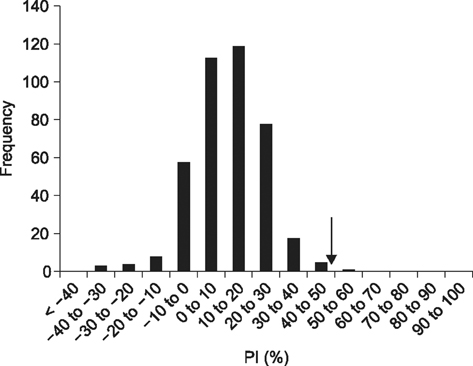
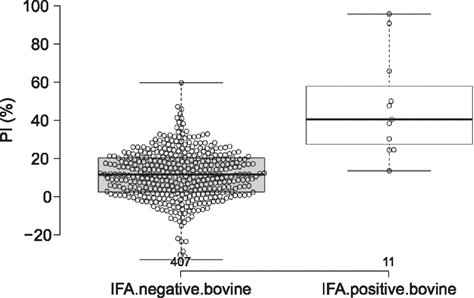
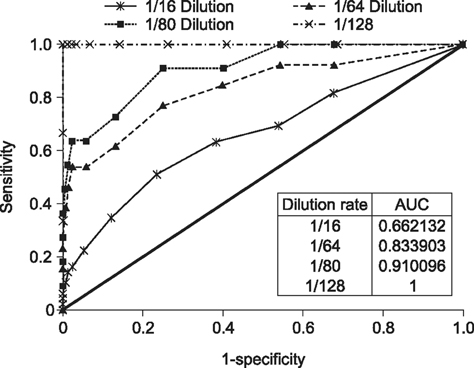
- Severe fever with thrombocytopenia syndrome (SFTS) caused by the SFTS virus (SFTSV), a phlebovirus in the family Bunyaviridae, is an emerging tick-borne infectious disease that impacts humans. This disease manifests as a decreased blood cell count and multi-organ failure, with a case-fatality rate of more than 12% in China. Because vaccines or antiviral drugs for the treatment of this disease are not available, monitoring the SFTS circulation in animals and controlling the tick-mammal cycle are important for preventing SFTS. Monoclonal antibodies against the recombinant nucleoprotein of SFTSV were generated to develop a competitive enzyme-linked immunosorbent assay (cELISA) for the detection of antibodies against SFTSV infection in cattle. The specificity and sensitivity of cELISA was assessed by comparing the results of this assay to those of an immunofluorescence assay (IFA). The results of the cELISA using 416 field bovine serum samples and laboratory-immunized positive sera showed 98.1% consistency with those of the IFA. The cELISA used in this study did not show cross-reactivity with antisera against other viral cattle diseases. The cELISA presented in this study can be applied to detect antibodies against SFTSV in cattle.
Keyword
MeSH Terms
-
Animals
Antibodies, Monoclonal/*blood
Antibodies, Viral/*blood
Bunyaviridae Infections/diagnosis/*veterinary/virology
Cattle
Cattle Diseases/blood/*diagnosis/virology
Enzyme-Linked Immunosorbent Assay/*veterinary
Fluorescent Antibody Technique/veterinary
Phlebovirus/*immunology/isolation & purification
Antibodies, Monoclonal
Antibodies, Viral
Figure
Cited by 2 articles
-
A serological study of severe fever with thrombocytopenia syndrome using a virus neutralization test and competitive enzyme-linked immunosorbent assay
Hyojin Lee, Eun-Ju Kim, In-Soo Cho, Jae-Young Song, Jeong Soo Choi, Ji Youn Lee, Yeun-Kyung Shin
J Vet Sci. 2017;18(1):33-38. doi: 10.4142/jvs.2017.18.1.33.Evaluation of two different enzyme-linked immunosorbent assay for severe fever with thrombocytopenia syndrome virus diagnosis
Min-Ah Yu, Hye Won Jeong, Su-Jin Park, Young-Il Kim, Hyeok-Il Kwon, Eun-Ha Kim, Young-Jae Si, Kwang Min Yu, Norbert John Robles, Hae Jung Han, Young Ki Choi
Clin Exp Vaccine Res. 2018;7(1):82-86. doi: 10.7774/cevr.2018.7.1.82.
Reference
-
1. Baneth G. Tick-borne infections of animals and humans: a common ground. Int J Parasitol. 2014; 44:591–596.
Article2. de StGroth SF, Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980; 35:1–21.
Article3. Ding S, Yin H, Xu X, Liu G, Jiang S, Wang W, Han X, Liu J, Niu G, Zhang X, Yu XJ, Wang X. A cross-sectional survey of severe fever with thrombocytopenia syndrome virus infection of domestic animals in Laizhou city, Shandong province, China. Jpn J Infect Dis. 2014; 67:1–4.
Article4. Jiang XL, Wang XJ, Li JD, Ding SJ, Zhang QF, Qu J, Zhang S, Li C, Wu W, Jiang M, Liang MF, Bi ZQ, Li DX. Isolation, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals. Bing Du Xue Bao. 2012; 28:252–257.5. Jiao Y, Zeng X, Guo X, Qi X, Zhang X, Shi Z, Zhou M, Bao C, Zhang W, Xu Y, Wang H. Preparation and evaluation of recombinant severe fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J Clin Microbiol. 2012; 50:372–377.
Article6. Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013; 19:1892–1894.
Article7. Li Z, Qi X, Zhou M, Bao C, Hu J, Wu B, Wang S, Tan Z, Fu J, Shan J, Zhu Y, Tang F. A two-tube multiplex real-time RT-PCR assay for the detection of four hemorrhagic fever viruses: severe fever with thrombocytopenia syndrome virus, Hantaan virus, Seoul virus, and dengue virus. Arch Virol. 2013; 158:1857–1863.
Article8. Libeau G, Diallo A, Calvez D, Lefèvre PC. A competitive ELISA using anti-N monoclonal antibodies for specific detection of rinderpest antibodies in cattle and small ruminants. Vet Microbiol. 1992; 31:147–160.
Article9. Liu JW, Wen HL, Fang LZ, Zhang ZT, He ST, Xue ZF, Ma DQ, Zhang XS, Wang T, Yu H, Zhang Y, Zhao L, Yu XJ. Prevalence of SFTSV among Asian house shrews and rodents, China, January-August 2013. Emerg Infect Dis. 2014; 20:2126–2128.
Article10. Magurano F, Nicoletti L. Humoral response in Toscana virus acute neurologic disease investigated by viral-proteinspecific immunoassays. Clin Diagn Lab Immunol. 1999; 6:55–60.
Article11. Martín-Folgar R, Lorenzo G, Boshra H, Iglesias J, Mateos F, Borrego B, Brun A. Development and characterization of monoclonal antibodies against Rift Valley fever virus nucleocapsid protein generated by DNA immunization. MAbs. 2010; 2:275–284.
Article12. Park SW, Han MG, Yun SM, Park C, Lee WJ, Ryou J. Severe fever with thrombocytopenia syndrome virus, South Korea, 2013. Emerg Infect Dis. 2014; 20:1880–1882.
Article13. Park SW, Song BG, Shin EH, Yun SM, Han MG, Park MY, Park C, Ryou J. Prevalence of severe fever with thrombocytopenia syndrome virus in Haemaphysalis longicornis ticks in South Korea. Ticks Tick Borne Dis. 2014; 5:975–977.
Article14. Spitzer M, Wildenhain J, Rappsilber J, Tyers M. BoxPlot: a web tool for generation of box plots. Nat Methods. 2014; 11:121–122.15. Sun JM, Zhang YJ, Gong ZY, Zhang L, Lv HK, Lin JF, Chai CL, Ling F, Liu SL, Gu SP, Zhu ZH, Zheng XH, Lan YQ, Ding F, Huang WZ, Xu JR, Chen EF, Jiang JM. Seroprevalence of severe fever with thrombocytopenia syndrome virus in southeastern China and analysis of risk factors. Epidemiol Infect. 2015; 143:851–856.
Article16. Sun Y, Liang M, Qu J, Jin C, Zhang Q, Li J, Jiang X, Wang Q, Lu J, Gu W, Zhang S, Li C, Wang X, Zhan F, Yao W, Bi Z, Wang S, Li D. Early diagnosis of novel SFTS bunyavirus infection by quantitative real-time RT-PCR assay. J Clin Virol. 2012; 53:48–53.
Article17. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, Kamei T, Honda M, Ninomiya D, Sakai T, Senba T, Kaneyuki S, Sakaguchi S, Satoh A, Hosokawa T, Kawabe Y, Kurihara S, Izumikawa K, Kohno S, Azuma T, Suemori K, Yasukawa M, Mizutani T, Omatsu T, Katayama Y, Miyahara M, Ijuin M, Doi K, Okuda M, Umeki K, Saito T, Fukushima K, Nakajima K, Yoshikawa T, Tani H, Fukushi S, Fukuma A, Ogata M, Shimojima M, Nakajima N, Nagata N, Katano H, Fukumoto H, Sato Y, Hasegawa H, Yamagishi T, Oishi K, Kurane I, Morikawa S, Saijo M. The first identification and retrospective study of Severe Fever with Thrombocytopenia syndrome in Japan. J Infect Dis. 2014; 209:816–827.
Article18. Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001; 356:983–989.
Article19. Woolhouse MEJ, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science. 2001; 292:1109–1112.
Article20. Wu W, Zhang S, Qu J, Zhang Q, Li C, Li J, Jin C, Liang M, Li D. Simultaneous detection of IgG antibodies associated with viral hemorrhagic fever by a multiplexed Luminex-based immunoassay. Virus Res. 2014; 187:84–90.
Article21. Xu B, Liu L, Huang X, Ma H, Zhang Y, Du Y, Wang P, Tang X, Wang H, Kang K, Zhang S, Zhao G, Wu W, Yang Y, Chen H, Mu F, Chen W. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan province, China: discovery of a new bunyavirus. PLoS Pathog. 2011; 7:e1002369.
Article22. Yathi KK, Bhasker S, Chinnamma M. Determination of B cell epitopes and evaluation of antigen capture ELISA for the earlier diagnosis of CHIK virus using anti-rCHIK E1 rabbit antibodies. J Immunol Methods. 2013; 393:45–52.
Article23. Yu L, Zhang L, Sun L, Lu J, Wu W, Li C, Zhang Q, Zhang F, Jin C, Wang X, Bi Z, Li D, Liang M. Critical epitopes in the nucleocapsid protein of SFTS virus recognized by a panel of SFTS patients derived human monoclonal antibodies. PLoS One. 2012; 7:e38291.
Article24. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011; 364:1523–1532.
Article25. Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, Lee YJ, Park C, Han MG. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis. 2014; 20:1358–1361.
Article26. Zhang X, Liu Y, Zhao L, Li B, Yu H, Wen H, Yu XJ. An emerging hemorrhagic fever in China caused by a novel bunyavirus SFTSV. Sci China Life Sci. 2013; 56:697–700.
Article27. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993; 39:561–577.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A serological study of severe fever with thrombocytopenia syndrome using a virus neutralization test and competitive enzyme-linked immunosorbent assay
- Evaluation of two different enzyme-linked immunosorbent assay for severe fever with thrombocytopenia syndrome virus diagnosis
- Development and evaluation of an immunochromatographic assay using a gp51 monoclonal antibody for the detection of antibodies against the bovine leukemia virus
- Development of the Enzyme Immunoassay for the Detection of Anti-HSV-2 Antibody with HSV-2 Specific Monoclonal Antibody
- Study on the ELISA method using monoclonal antibody for the detection of Hantaan virus