J Vet Sci.
2016 Sep;17(3):269-277. 10.4142/jvs.2016.17.3.269.
Hydration status affects osteopontin expression in the rat kidney
- Affiliations
-
- 1Department of Anatomy, Ewha Womans University School of Medicine, Seoul 03760, Korea. khhan@ewha.ac.kr
- 2Division of Nephrology, College of Medicine, University of Florida, Gainesville, FL 32608, USA.
- 3Nephrology Section, North Florida/South Georgia Veterans Health System (NF/SGVHS), Gainesville, FL 32608, USA.
- 4Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2413125
- DOI: http://doi.org/10.4142/jvs.2016.17.3.269
Abstract
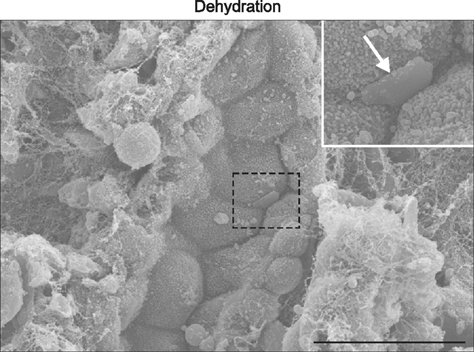
- Osteopontin (OPN) is a secretory protein that plays an important role in urinary stone formation. Hydration status is associated with the development of urolithiasis. This study was conducted to examine the effects of dehydration and hydration on OPN expression in the rat kidney. Animals were divided into three groups, control, dehydrated, and hydrated. Kidney tissues were processed for light and electron microscope immunocytochemistry, in situ hybridization, and immunoblot analysis. Dehydration induced a significant increase in OPN protein expression, whereas increased fluid intake induced a decrease in protein expression. Under control conditions, OPN protein and mRNA expression were only detected in the descending thin limb (DTL). Dehydration induced increased expression in the DTL and the development of detectable expression in the thick ascending limb (TAL). In contrast, OPN expression levels declined to less than the controls in the DTL after hydration, while no expression of either protein or mRNA was detectable in the TAL. Immunoelectron microscopy demonstrated that hydration status altered tubular ultrastructure and intracellular OPN expression in the Golgi apparatus and secretory cytoplasmic vesicles. These data confirm that changes in oral fluid intake can regulate renal tubular epithelial cell OPN expression.
Keyword
MeSH Terms
Figure
Reference
-
1. Asplin JR, Arsenault D, Parks JH, Coe FL, Hoyer JR. Contribution of human uropontin to inhibition of calcium oxalate crystallization. Kidney Int. 1998; 53:194–199.
Article2. Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol. 1996; 155:839–843.
Article3. Borghi L, Meschi T, Amato F, Novarini A, Romanelli A, Cigala F. Hot occupation and nephrolithiasis. J Urol. 1993; 150:1757–1760.
Article4. Choi S, Kim JA, Na HY, Kim JE, Park S, Han KH, Kim YJ, Suh SH. NADPH oxidase 2-derived superoxide downregulates endothelial KCa3.1 in preeclampsia. Free Radic Biol Med. 2013; 57:10–21.
Article5. Diamond JR, Kees-Folts D, Ricardo SD, Pruznak A, Eufemio M. Early and persistent up-regulated expression of renal cortical osteopontin in experimental hydronephrosis. Am J Pathol. 1995; 146:1455–1466.6. Embon OM, Rose GA, Rosenbaum T. Chronic dehydration stone disease. Br J Urol. 1990; 66:357–362.
Article7. Giachelli CM, Pichler R, Lombardi D, Denhardt DT, Alpers CE, Schwartz SM, Johnson RJ. Osteopontin expression in angiotensin II-induced tubulointerstitial nephritis. Kidney Int. 1994; 45:515–524.
Article8. Hamamoto S, Nomura S, Yasui T, Okada A, Hirose M, Shimizu H, Itoh Y, Tozawa K, Kohri K. Effects of impaired functional domains of osteopontin on renal crystal formation: analyses of OPN transgenic and OPN knockout mice. J Bone Miner Res. 2010; 25:2712–2723.9. Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, rh C glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol. 2006; 17:2670–2679.
Article10. Han KH, Jung JY, Cha JH, Kim H, Madsen KM, Kim J. 1,25-Dihydroxyvitamin D3 stimulates osteopontin expression in rat kidney. Nephron Physiol. 2003; 93:76–86.11. Han KH, Lee HW, Handlogten ME, Bishop JM, Levi M, Kim J, Verlander JW, Weiner ID. Effect of hypokalemia on renal expression of the ammonia transporter family members, Rh B glycoprotein and Rh C glycoprotein, in the rat kidney. Am J Physiol Renal Physiol. 2011; 301:F823–F832.
Article12. Kleinman JG, Beshensky A, Worcester EM, Brown D. Expression of osteopontin, a urinary inhibitor of stone mineral crystal growth, in rat kidney. Kidney Int. 1995; 47:1585–1596.
Article13. Kohri K, Nomura S, Kitamura Y, Nagata T, Yoshioka K, Iguchi M, Yamate T, Umekawa T, Suzuki Y, Sinohara H, Kurita T. Structure and expression of the mRNA encoding urinary stone protein (osteopontin). J Biol Chem. 1993; 268:15180–15184.
Article14. Kohri K, Suzuki Y, Yoshida K, Yamamoto K, Amasaki N, Yamate T, Umekawa T, Iguchi M, Sinohara H, Kurita T. Molecular cloning and sequencing of cDNA encoding urinary stone protein, which is identical to osteopontin. Biochem Biophys Res Commun. 1992; 184:859–864.
Article15. Kohri K, Yasui T, Okada A, Hirose M, Hamamoto S, Fujii Y, Niimi K, Taguchi K. Biomolecular mechanism of urinary stone formation involving osteopontin. Urol Res. 2012; 40:623–637.
Article16. Konya E, Umekawa T, Iguchi M, Kurita T. The role of osteopontin on calcium oxalate crystal formation. Eur Urol. 2003; 43:564–571.
Article17. Lee SY, Han SM, Kim JE, Chung KY, Han KH. Expression of E-cadherin in pig kidney. J Vet Sci. 2013; 14:381–386.
Article18. Lee SY, Shin JA, Kwon HM, Weiner ID, Han KH. Renal ischemia-reperfusion injury causes intercalated cell-specific disruption of occludin in the collecting duct. Histochem Cell Biol. 2011; 136:637–647.
Article19. Ma G, Young DB, Clower BR, Anderson PG, Lin H, Abide AM. High potassium intake inhibits neointima formation in the rat carotid artery balloon injury model. Am J Hypertens. 2000; 13:1014–1020.
Article20. Manz F, Wentz A. The importance of good hydration for the prevention of chronic diseases. Nutr Rev. 2005; 63(6 Pt 2):S2–S5.
Article21. Mazzali M, Hughes J, Dantas M, Liaw L, Steitz S, Alpers CE, Pichler RH, Lan HY, Giachelli CM, Shankland SJ, Couser WG, Johnson RJ. Effects of cyclosporine in osteopontin null mice. Kidney Int. 2002; 62:78–85.22. McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res. 1995; 10:1913–1929.
Article23. Okada A, Nomura S, Saeki Y, Higashibata Y, Hamamoto S, Hirose M, Itoh Y, Yasui T, Tozawa K, Kohri K. Morphological conversion of calcium oxalate crystals into stones is regulated by osteopontin in mouse kidney. J Bone Miner Res. 2008; 23:1629–1637.
Article24. Okada H, Moriwaki K, Kalluri R, Takenaka T, Imai H, Ban S, Takahama M, Suzuki H. Osteopontin expressed by renal tubular epithelium mediates interstitial monocyte infiltration in rats. Am J Physiol Renal Physiol. 2000; 278:F110–F121.
Article25. Ophascharoensuk V, Giachelli CM, Gordon K, Hughes J, Pichler R, Brown P, Liaw L, Schmidt R, Shankland SJ, Alpers CE, Couser WG, Johnson RJ. Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 1999; 56:571–580.
Article26. Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int. 2003; 63:543–553.
Article27. Persy VP, Verstrepen WA, Ysebaert DK, De Greef KE, De Broe ME. Differences in osteopontin up-regulation between proximal and distal tubules after renal ischemia/reperfusion. Kidney Int. 1999; 56:601–611.
Article28. Pichler R, Giachelli C, Young B, Alpers CE, Couser WG, Johnson RJ. The pathogenesis of tubulointerstitial disease associated with glomerulonephritis: the glomerular cytokine theory. Miner Electrolyte Metab. 1995; 21:317–327.29. Pichler R, Giachelli CM, Lombardi D, Pippin J, Gordon K, Alpers CE, Schwartz SM, Johnson RJ. Tubulointerstitial disease in glomerulonephritis. Potential role of osteopontin (uropontin). Am J Pathol. 1994; 144:915–926.30. Rule AD, Bergstralh EJ, Melton LJ 3rd, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009; 4:804–811.
Article31. Shiraga H, Min W, VanDusen WJ, Clayman MD, Miner D, Terrell CH, Sherbotie JR, Foreman JW, Przysiecki C, Neilson EG. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A. 1992; 89:426–430.
Article32. Tsuji H, Shimizu N, Nozawa M, Umekawa T, Yoshimura K, De Velasco MA, Uemura H, Khan SR. Osteopontin knockdown in the kidneys of hyperoxaluric rats leads to reduction in renal calcium oxalate crystal deposition. Urolithiasis. 2014; 42:195–202.
Article33. Umekawa T, Kohri K, Kurita T, Hirota S, Nomura S, Kitamura Y. Expression of osteopontin messenger RNA in the rat kidney on experimental model of renal stone. Biochem Mol Biol Int. 1995; 35:223–230.34. Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003; 14:139–147.
Article35. Worcester EM, Blumenthal SS, Beshensky AM, Lewand DL. The calcium oxalate crystal growth inhibitor protein produced by mouse kidney cortical cells in culture is osteopontin. J Bone Miner Res. 1992; 7:1029–1036.
Article36. Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M, Gejyo F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001; 60:1645–1657.
Article37. Yamate T, Kohri K, Umekawa T, Amasaki N, Amasaki N, Isikawa Y, Iguchi M, Kurita T. The effect of osteopontin on the adhesion of calcium oxalate crystals to Madin-Darby canine kidney cells. Eur Urol. 1996; 30:388–393.
Article38. Yamate T, Kohri K, Umekawa T, Iguchi M, Kurita T. Osteopontin antisense oligonucleotide inhibits adhesion of calcium oxalate crystals in Madin-Darby canine kidney cell. J Urol. 1998; 160:1506–1512.
Article39. Yasui T, Fujita K, Asai K, Kohri K. Osteopontin regulates adhesion of calcium oxalate crystals to renal epithelial cells. Int J Urol. 2002; 9:100–108.
Article40. Yu XQ, Nikolic-Paterson DJ, Mu W, Giachelli CM, Atkins RC, Johnson RJ, Lan HY. A functional role for osteopontin in experimental crescentic glomerulonephritis in the rat. Proc Assoc Am Physicians. 1998; 110:50–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osteopontin and Developing Kidney
- Dehydration Increases Osteopontin Expression in Rat Kidney
- Expression of Osteopontin in the Adult and Developing Rat Kidney
- Role of zinc for calcification inhibitor protein in vascular smooth muscle cell plaque formation
- Decreased osteopontin expression in the rat kidney on a sodium deficient diet