Yonsei Med J.
2018 Jul;59(5):643-651. 10.3349/ymj.2018.59.5.643.
Effects and Predictive Factors of Immunosuppressive Therapy Combined with Umbilical Cord Blood Infusion in Patients with Severe Aplastic Anemia
- Affiliations
-
- 1Department of Hematology, Taihe Hospital Affiliated to Hubei University of Medicine, Shiyan, China. chuchengwan@126.com
- KMID: 2412655
- DOI: http://doi.org/10.3349/ymj.2018.59.5.643
Abstract
- PURPOSE
To investigate the efficacy and safety of umbilical cord blood (UCB) infusion (UCBI) plus immunosuppressive therapy (IST) treatment in comparison to IST treatment, as well as predictive factors for clinical responses, in severe aplastic anemia (SAA) patients.
MATERIALS AND METHODS
Totally, 93 patients with SAA were enrolled in this cohort study. In the IST group, rabbit antithymocyte globulin (r-ATG) combined with cyclosporine A (CsA) was administered, while in the IST+UBCI group, r-ATG, CsA, and UCB were used.
RESULTS
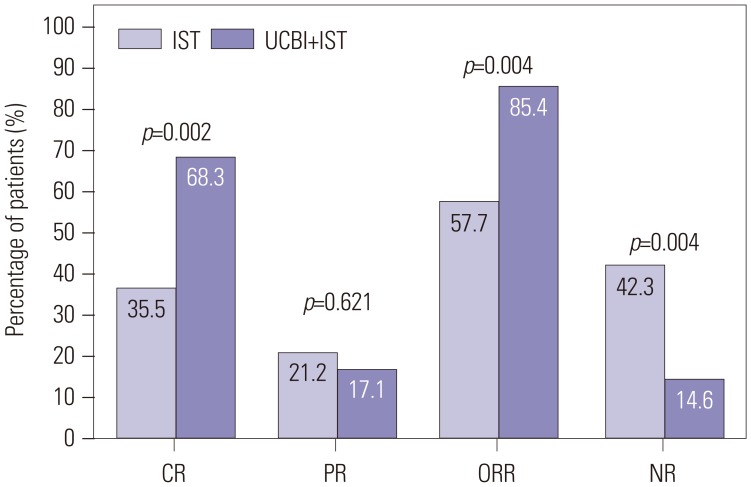
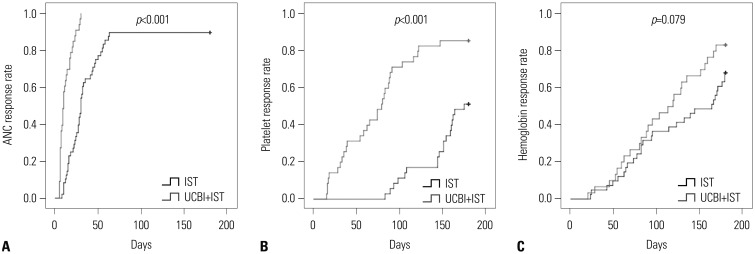
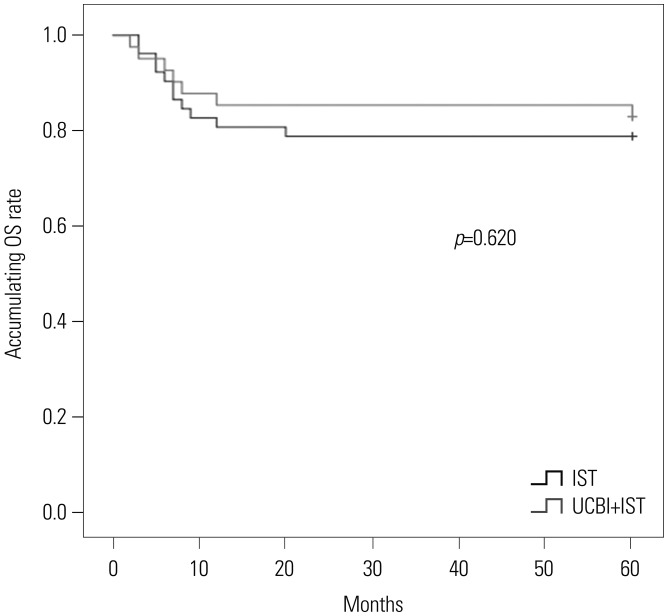
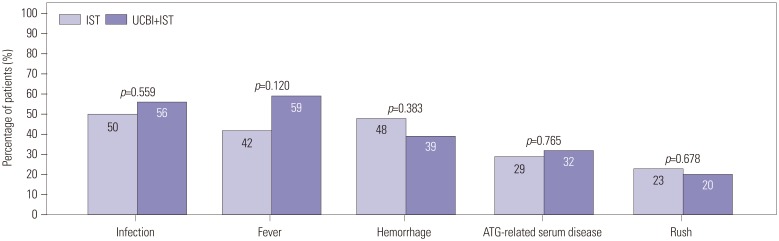
After 6 months of treatment, UCBI+IST achieved a higher complete response (CR) rate (p=0.002) and an elevated overall response rate (ORR) (p=0.004), compared to IST. Regarding hematopoietic recovery at month 6, platelet responses in the UCBI+IST group were better than those in the IST group (p=0.002), and UCBI+IST treatment facilitated increasing trends in absolute neutrophil count (ANC) response (p=0.056). Kaplan-Meier curves illuminated UCBI+IST achieved faster ANC response (p < 0.001) and platelet response (p < 0.001), compared with IST therapy. There was no difference in overall survival (OS) between the two groups (p=0.620). Furthermore, logistic regression analysis demonstrated that UCBI+IST was an independent predicting factor for both CR (p=0.001) and ORR (p < 0.001), compared to IST; meanwhile, very severe aplastic anemia (VSAA) and ANC could predict clinical responses as well. However, Cox proportional hazard regression indicated that VSAA (p=0.003), but not UCBI+IST, affected OS. Safety profiles showed that UCBI+IST therapy did not elevate adverse events, compared with IST treatment.
CONCLUSION
UCBI+IST achieved better clinical responses and hematopoietic recovery than IST, and was well tolerated in SAA patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Yu Z, Zhou F, Ge LF, Liu XM, Fang Y, Xie L, et al. High-dose immunosuppressive therapy combined with cord blood infusion and non-myeloablative peripheral blood stem cell transplantation for patients with severe aplastic anemia. Eur Rev Med Pharmacol Sci. 2013; 17:2613–2618. PMID: 24142608.2. Li Y, Sheng Z, Niu S, Ge L, Ren C, Zou Y. Rapid and complete reconstitution of autologous haemopoiesis after cord blood infusion in treatment-naive patients with severe aplastic anemia receiving high-dose cyclophosphamide/ATG therapy. Eur J Haematol. 2013; 90:45–50. PMID: 23106334.
Article3. Xie LN, Fang Y, Yu Z, Song NX, Kong FS, Liu XM, et al. Increased immunosuppressive treatment combined with unrelated umbilical cord blood infusion in children with severe aplastic anemia. Cell Immunol. 2014; 289:150–154. PMID: 24838091.
Article4. Kurre P, Johnson FL, Deeg HJ. Diagnosis and treatment of children with aplastic anemia. Pediatr Blood Cancer. 2005; 45:770–780. PMID: 15706582.
Article5. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006; 108:2509–2519. PMID: 16778145.
Article6. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012; 120:1185–1196. PMID: 22517900.
Article7. Füreder W, Valent P. Treatment of refractory or relapsed acquired aplastic anemia: review of established and experimental approaches. Leuk Lymphoma. 2011; 52:1435–1445. PMID: 21635205.
Article8. Brodsky RA, Chen AR, Dorr D, Fuchs EJ, Huff CA, Luznik L, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010; 115:2136–2141. PMID: 20018919.
Article9. Hattori M, Terasawa T, Tsushita K, Utsumi M, Kawano F, Saito H, et al. The status of antithymocyte globulin therapy for adult patients in Japan: retrospective analysis of a nationwide survey. Int J Hematol. 2008; 87:48–55. PMID: 18224413.
Article10. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989; 321:1174–1178. PMID: 2571931.
Article11. Mao P, Wang S, Wang S, Zhu Z, Liv Q, Xuv Y, et al. Umbilical cord blood transplant for adult patients with severe aplastic anemia using anti-lymphocyte globulin and cyclophosphamide as conditioning therapy. Bone Marrow Transplant. 2004; 33:33–38. PMID: 14704655.
Article12. Rosenthal J, Woolfrey AE, Pawlowska A, Thomas SH, Appelbaum F, Forman S. Hematopoietic cell transplantation with autologous cord blood in patients with severe aplastic anemia: an opportunity to revisit the controversy regarding cord blood banking for private use. Pediatr Blood Cancer. 2011; 56:1009–1012. PMID: 21370429.
Article13. Auerbach AD. Umbilical cord blood transplants for genetic disease: diagnostic and ethical issues in fetal studies. Blood Cells. 1994; 20:303–309. PMID: 7749111.14. Eapen M, Rubinstein P, Zhang MJ, Camitta BM, Stevens C, Cairo MS, et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006; 24:145–151. PMID: 16382124.
Article15. Camitta BM, Storb R, Thomas ED. Aplastic anemia (first of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982; 306:645–652. PMID: 7035946.16. Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988; 70:177–182. PMID: 3056497.
Article17. Camitta BM. What is the definition of cure for aplastic anemia? Acta Haematol. 2000; 103:16–18. PMID: 10705154.
Article18. Ustun C, Giannotti F, Zhang MJ, Wang HL, Brunstein C, Labopin M, et al. Outcomes of UCB transplantation are comparable in FLT3+ AML: results of CIBMTR, EUROCORD and EBMT collaborative analysis. Leukemia. 2017; 31:1408–1414. PMID: 28119528.
Article19. Raut S, Shah S, Shah K, Patel K, Talati S, Parikh S, et al. Improving outcome of thalassemia with hematopoetic stem cell transplantation: an experience of Gujarat Cancer Research Institute. Indian J Hematol Blood Transfus. 2016; 32:284–291. PMID: 27429520.
Article20. MacMillan ML, DeFor TE, Young JA, Dusenbery KE, Blazar BR, Slungaard A, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015; 125:3798–3804. PMID: 25824692.
Article21. Xu LX, Cao YB, Liu ZY, Wu YM, Wang ZH, Yan B, et al. [Transplantation of haploidentical-hematopoietic stem cells combined with two kind of third part cells for chronic aplastic anemia: one case report]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013; 21:1522–1525. PMID: 24370041.22. Huang X, Liu D. Related HLA-mismatched/haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion: observations of a single Chinese center. Clin Transpl. 2011; 237–245. PMID: 22755417.23. Baron F, Nagler A. Novel strategies for improving hematopoietic reconstruction after allogeneic hematopoietic stem cell transplantation or intensive chemotherapy. Expert Opin Biol Ther. 2017; 17:163–174. PMID: 27927023.
Article24. Azuma H, Watanabe E, Otsuka Y, Negishi Y, Ohkura S, Shinya E, et al. Induction of langerin+ Langerhans cell-like cells expressing reduced TLR3 from CD34+ cord blood cells stimulated with GM-CSF, TGF-β1, and TNF-α. Biomed Res. 2016; 37:271–281. PMID: 27784870.25. Liu Y, Chen XH, Si YJ, Li ZJ, Gao L, Gao L, et al. Reconstruction of hematopoietic inductive microenvironment after transplantation of VCAM-1-modified human umbilical cord blood stromal cells. PLoS One. 2012; 7:e31741. PMID: 22384064.
Article26. Liu Y, Yi L, Wang L, Chen L, Chen X, Wang Y. Ginsenoside Rg1 protects human umbilical cord blood-derived stromal cells against tert-Butyl hydroperoxide-induced apoptosis through Akt-FoxO3a-Bim signaling pathway. Mol Cell Biochem. 2016; 421:75–87. PMID: 27522666.
Article27. Li J, Wong WH, Chan S, Chim JC, Cheung KM, Lee TL, et al. Factors affecting mesenchymal stromal cells yield from bone marrow aspiration. Chin J Cancer Res. 2011; 23:43–48. PMID: 23467386.
Article28. Sanz J, Arango M, Carpio N, Montesinos P, Moscardó F, Martín G, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single-center experience. Bone Marrow Transplant. 2014; 49:1084–1088. PMID: 24887383.
Article29. Miano M, Dufour C. The diagnosis and treatment of aplastic anemia: a review. Int J Hematol. 2015; 101:527–535. PMID: 25837779.
Article30. Peffault de Latour R, Rocha V, Socié G. Cord blood transplantation in aplastic anemia. Bone Marrow Transplant. 2013; 48:201–202. PMID: 23292234.
Article31. Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008; 15:162–168. PMID: 18391779.
Article32. Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA. 2010; 304:1358–1364. PMID: 20858879.
Article33. Red Blood Cell Disease (Anemia) Group. Chinese Society of Hematology. Chinese Medical Association. Chinese expert consensus on the diagnosis and treatment of aplastic anemia (2017). Zhonghua Xue Ye Xue Za Zhi. 2017; 38:1–5. PMID: 28219216.34. Ohga S, Ichino K, Goto K, Hattori S, Nomura A, Takada H, et al. Unrelated donor cord blood transplantation for childhood severe aplastic anemia after a modified conditioning. Pediatr Transplant. 2006; 10:497–500. PMID: 16712610.
Article35. Ruangkanchanasetr P, Srichaikul T, Supaporn T. Triple immunosuppressive therapy can accelerate the recovery of antibody-mediated pure red cell aplasia and allow successful concurrent resumption of erythropoietin. J Med Assoc Thai. 2012; 95(Suppl 5):S92–S95.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Salvage Unrelated Umbilical Cord Blood Transplantation with Two Units After Engraftment Failure with Single Unit in Severe Aplastic Anemia
- Therapy of severe aplastic anemia with anti-human thymocyte globulin (ATGAM) with and without HLA-haploidentical bone-marrow infusion
- Successful engraftment after infusion of multiple low doses of CD34+ cells from a poorly matched sibling donor in a patient with severe aplastic anemia
- Papillary Thyroid Cancer in a Child with Very Severe Aplastic Anemia
- Two Cases of Aplastic Anemia Following Propylthiouracil