J Vet Sci.
2016 Dec;17(4):587-589. 10.4142/jvs.2016.17.4.587.
First detection of West Nile virus in domestic pigeon in Korea
- Affiliations
-
- 1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. pjhak@snu.ac.kr
- 2Biological Resources Research Department, National Institute of Biological Resources, Incheon 22689, Korea.
- 3Environmental Health Research Department, National Institute of Environmental Research, Incheon 22689, Korea.
- 4Department of Wildlife Animal Medicine, College of Veterinary Medicine, Kangwon National University, Chuncheon 24341, Korea.
- 5Department of Science Education, Jeju National University, Jeju 63243, Korea.
- KMID: 2412617
- DOI: http://doi.org/10.4142/jvs.2016.17.4.587
Abstract
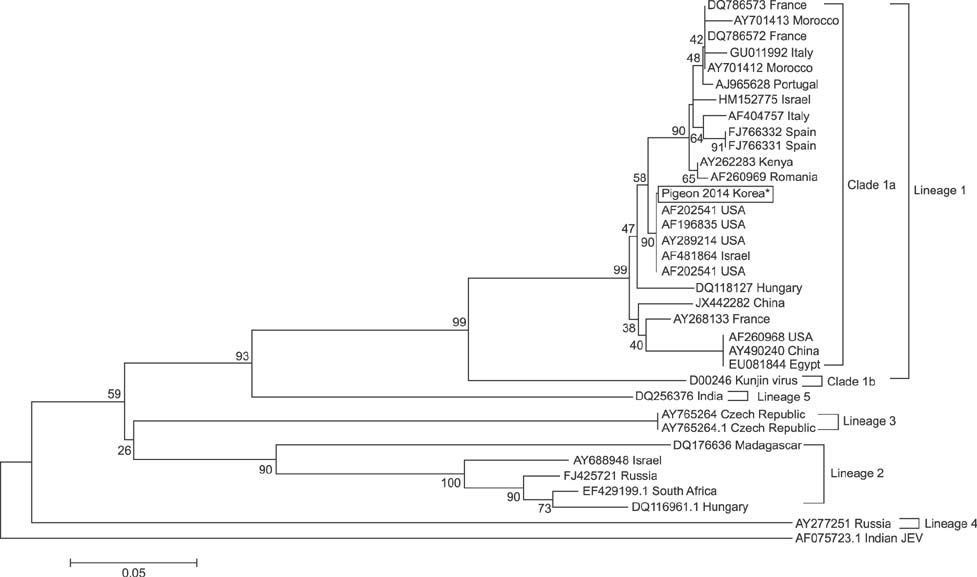
- West Nile virus (WNV) is a mosquito-borne zoonotic pathogen that has spread throughout Europe and the United States. Recently, WNV spread to East and Southeast Asia, and great efforts have been made in South Korea to prevent the spread of WNV from neighboring countries. In this study, we diagnosed the first case of WNV in pigeons (Columba livia domestica) residing in cities using a competitive enzyme-linked immunosorbent assay and confirmed it with nested reverse transcription polymerase chain reaction analysis and sequencing. This is the first report to provide convincing evidence that WNV is present within South Korea.
Keyword
MeSH Terms
-
Alternative Splicing
Animals
Bird Diseases/*epidemiology/virology
Case-Control Studies
*Columbidae
Enzyme-Linked Immunosorbent Assay/veterinary
Polymerase Chain Reaction
Republic of Korea/epidemiology
Reverse Transcriptase Polymerase Chain Reaction/veterinary
West Nile Fever/epidemiology/microbiology/*veterinary
West Nile virus/*isolation & purification
Figure
Reference
-
1. Chaintoutis SC, Dovas CI, Papanastassopoulou M, Gewehr S, Danis K, Beck C, Lecollinet S, Antalis V, Kalaitzopoulou S, Panagiotopoulos T, Mourelatos S, Zientara S, Papadopoulos O. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comp Immunol Microbiol Infect Dis. 2014; 37:131–141.
Article2. Ciota AT, Kramer LD. Vector-virus interactions and transmission dynamics of West Nile virus. Viruses. 2013; 5:3021–3047.
Article3. Hwang J, Ryu HS, Kim H, Lee SA. The first reported case of West Nile encephalitis in Korea. J Korean Med Sci. 2015; 30:343–345.
Article4. Johnson DJ, Ostlund EN, Pedersen DD, Schmitt BJ. Detection of North American West Nile virus in animal tissue by a reverse transcription-nested polymerase chain reaction assay. Emerg Infect Dis. 2001; 7:739–741.
Article5. Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001; 7:621–625.
Article6. Panella NA, Young G, Komar N. Experimental infection of Eurasian collared-dove (Streptopelia decaocto) with West Nile virus. J Vector Ecol. 2013; 38:210–214.
Article7. Sambri V, Capobianchi M, Charrel R, Fyodorova M, Gaibani P, Gould E, Niedrig M, Papa A, Pierro A, Rossini G, Varani S, Vocale C, Landini MP. West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin Microbiol Infect. 2013; 19:699–704.
Article8. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28:2731–2739.
Article9. Wheeler SS, Vineyard MP, Woods LW, Reisen WK. Dynamics of West Nile virus persistence in House Sparrows (Passer domesticus). PLoS Negl Trop Dis. 2012; 6:e1860.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuropsychological and Psychiatric Impairment after West Nile Virus Encephalitis in Korean: A Case Report
- The First Reported Case of West Nile Encephalitis in Korea
- Serological evidence of West Nile viral infection in archived swine serum samples from Peninsular Malaysia
- Ecological characteristics and current status of infectious disease vectors in South Korea
- Emerging Infectious Disease and Safety of Blood Components