J Vet Sci.
2017 Mar;18(1):17-23. 10.4142/jvs.2017.18.1.17.
Expression of von Willebrand factor, pulmonary intravascular macrophages, and Toll-like receptors in lungs of septic foals
- Affiliations
-
- 1Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, SK S7N 5B4, Canada. baljit.singh1@ucalgary.ca
- 2Department of Veterinary Pathology, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, SK S7N 5B4, Canada.
- KMID: 2412583
- DOI: http://doi.org/10.4142/jvs.2017.18.1.17
Abstract
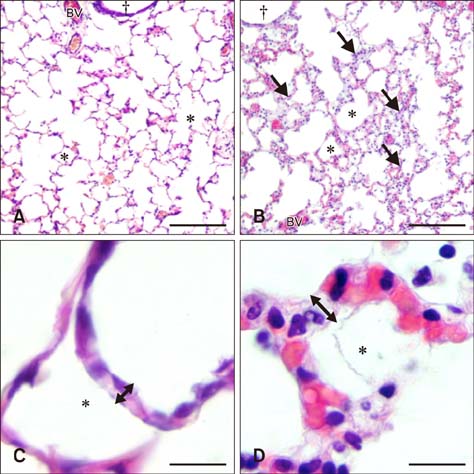
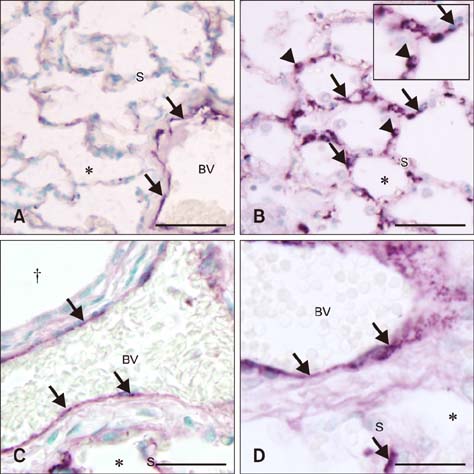
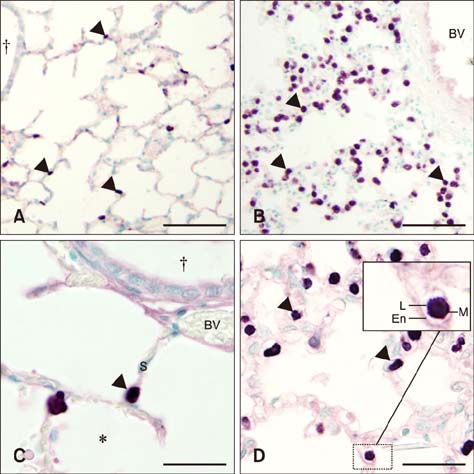
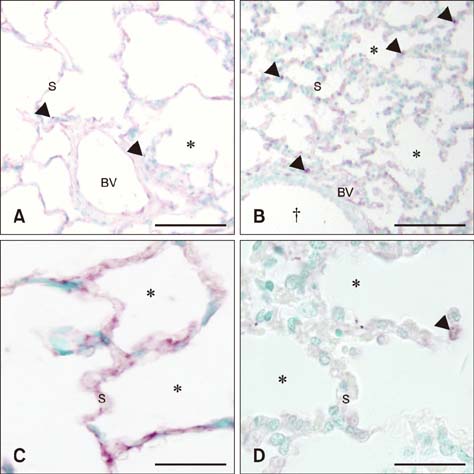
- Sepsis causes significant mortality in neonatal foals; however, there is little data describing the cellular and molecular pathways of lung inflammation in septic foals. This study was conducted to characterize lung inflammation in septic foals. Lung tissue sections from control (n = 6) and septic (n = 17) foals were compared using histology and immunohistology. Blinded pathologic scoring of hematoxylin and eosin stained samples revealed increased features of lung inflammation such as thickened alveolar septa and sequestered inflammatory cells in septic foals. Septic foal lungs showed increased expression of von Willebrand factor in blood vessels, demonstrating vascular inflammation. Use of MAC387 antibody to detect calprotectin as a reflection of mononuclear cell infiltration revealed a significant increase in their numbers in alveolar septa of lungs from septic foals compared to those from control foals. The mononuclear cells appeared to be mature macrophages and were located in the septal capillaries, suggesting they were pulmonary intravascular macrophages (PIMs). Finally, lungs from septic foals showed increased expression of Toll-like receptor 4 and 9 in mononuclear cells relative to the control. Taken together, this study is the first to show the expression of inflammatory molecules and an increase in PIMs in lungs from foals that died from sepsis.
Keyword
MeSH Terms
-
Animals
*Gene Expression
Horse Diseases/*genetics/immunology/microbiology
Horses
Inflammation/genetics/immunology/microbiology/*veterinary
Lung/metabolism
Macrophages, Alveolar/*metabolism
Sepsis/genetics/immunology/microbiology/*veterinary
Toll-Like Receptors/*genetics/metabolism
von Willebrand Factor/*genetics/metabolism
Toll-Like Receptors
von Willebrand Factor
Figure
Reference
-
1. Atwal OS. Pulmonary intravascular macrophages (PIMs) and cationised ferritin-induced phagocytosis of platelets: a morphological study. Comp Haematol Int. 1992; 2:179–185.
Article2. Brewer BD, Koterba AM. Development of a scoring system for the early diagnosis of equine neonatal sepsis. Equine Vet J. 1988; 20:18–22.
Article3. Charavaryamath C, Janardhan KS, Caldwell S, Singh B. Pulmonary intravascular monocytes/macrophages in a rat model of sepsis. Anat Rec A Discov Mol Cell Evol Biol. 2006; 288:1259–1271.
Article4. Cunningham AJ. Acute respiratory distress syndrome—two decades later. Yale J Biol Med. 1991; 64:387–402.5. DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009; 30:547–556.
Article6. Dunkel B, Dolente B, Boston RC. Acute lung injury/acute respiratory distress syndrome in 15 foals. Equine Vet J. 2005; 37:435–440.
Article7. Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011; 9:Suppl 1. 182–188.
Article8. Frevert CW, Warner AE. Respiratory distress resulting from acute lung injury in the veterinary patient. J Vet Intern Med. 1992; 6:154–165.
Article9. Gill SS, Suri SS, Janardhan KS, Caldwell S, Duke T, Singh B. Role of pulmonary intravascular macrophages in endotoxin-induced lung inflammation and mortality in a rat model. Respir Res. 2008; 9:69.
Article10. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006; 19:788–802.
Article11. Janardhan KS, McIsaac M, Fowlie J, Shrivastav A, Caldwell S, Sharma RK, Singh B. Toll like receptor-4 expression in lipopolysaccharide induced lung inflammation. Histol Histopathol. 2006; 21:687–696.12. Johnson L, Montgomery JB, Schneider JP, Townsend HGG, Ochs M, Singh B. Morphometric examination of the equine adult and foal lung. Anat Rec (Hoboken). 2014; 297:1950–1962.
Article13. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013; 13:159–175.
Article14. Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015; 125:2019–2028.
Article15. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009; 22:240–273.
Article16. Morris DD. Endotoxemia in horses: a review of cellular and humoral mediators involved in its pathogenesis. J Vet Intern Med. 1991; 5:167–181.
Article17. Parbhakar OP, Duke T, Townsend HGG, Singh B. Depletion of pulmonary intravascular macrophages partially inhibits lipopolysaccharide-induced lung inflammation in horses. Vet Res. 2005; 36:557–569.
Article18. Raisis AL, Hodgson JL, Hodgson DR. Equine neonatal septicaemia: 24 cases. Aust Vet J. 1996; 73:137–140.
Article19. Roy MF. Sepsis in adults and foals. Vet Clin North Am Equine Pract. 2004; 20:41–61.
Article20. Sanchez LC. Equine neonatal sepsis. Vet Clin North Am Equine Pract. 2005; 21:273–293.
Article21. Schneberger D, Aharonson-Raz K, Singh B. Pulmonary intravascular macrophages and lung health: what are we missing? Am J Physiol Lung Cell Mol Physiol. 2012; 302:L498–L503.
Article22. Schneberger D, Caldwell S, Suri SS, Singh B. Expression of Toll-like receptor 9 in horse lungs. Anat Rec (Hoboken). 2009; 292:1068–1077.
Article23. Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, Mele T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res Notes. 2014; 7:233.
Article24. Singh B, Pearce JW, Gamage LN, Janardhan K, Caldwell S. Depletion of pulmonary intravascular macrophages inhibits acute lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2004; 286:L363–L372.
Article25. Taylor S. A review of equine sepsis. Equine Vet Educ. 2015; 27:99–109.
Article26. Theelen MJP, Wilson WD, Edman JM, Magdesian KG, Kass PH. Temporal trends in prevalence of bacteria isolated from foals with sepsis: 1979-2010. Equine Vet J. 2014; 46:169–173.
Article27. Wassef A, Janardhan K, Pearce JW, Singh B. Toll-like receptor 4 in normal and inflamed lungs and other organs of pig, dog and cattle. Histol Histopathol. 2004; 19:1201–1208.28. Weber EJ, Sanchez LC, Giguère S. Re-evaluation of the sepsis score in equine neonates. Equine Vet J. 2015; 47:275–278.
Article29. Wilkins PA, Otto CM, Baumgardner JE, Dunkel B, Bedenice D, Paradis MR, Staffieri F, Syring RS, Slack J, Grasso S, Pranzo EG. Acute lung injury and acute respiratory distress syndromes in veterinary medicine: consensus definitions: The Dorothy Russell Havemeyer Working Group on ALI and ARDS in Veterinary Medicine. J Vet Emerg Crit Care. 2007; 17:333–339.
Article30. Wilson RF, Sibbald WJ. Acute respiratory failure. Crit Care Med. 1976; 4:79–89.
Article31. Winkler GC. Pulmonary intravascular macrophages in domestic animal species: review of structural and functional properties. Am J Anat. 1988; 181:217–234.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laboratory assessment of von Willebrand factor for classification of von Willebrand disease
- Three cases of type I von Willebrand disease in a family
- Detection of Novel C4517G (Ser743Trp) Mutation in a Family with Type 2A von Willebrand Disease
- Effect of immune-mediated vascular injury on the coagulation- regulatory mechanism of the human endothelial cells; changes of tissue-type plasminogen activator, plasminogen activator inhibitor- 1 and von Willebrand factor
- von Willebrand Disease