J Vet Sci.
2017 Jun;18(2):193-200. 10.4142/jvs.2017.18.2.193.
Alterations in antioxidant function and cell apoptosis in duck spleen exposed to molybdenum and/or cadmium
- Affiliations
-
- 1Institute of Animal Population Health, College of Animal Science and Technology, Jiangxi Agriculture University, Nanchang 330045, China. zhangcaiying0916@163.com
- KMID: 2412572
- DOI: http://doi.org/10.4142/jvs.2017.18.2.193
Abstract
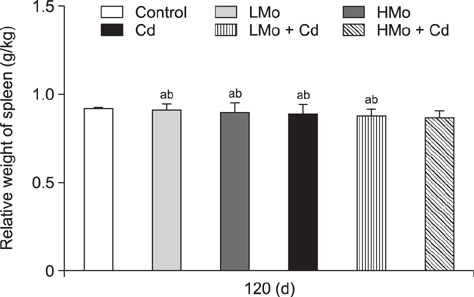
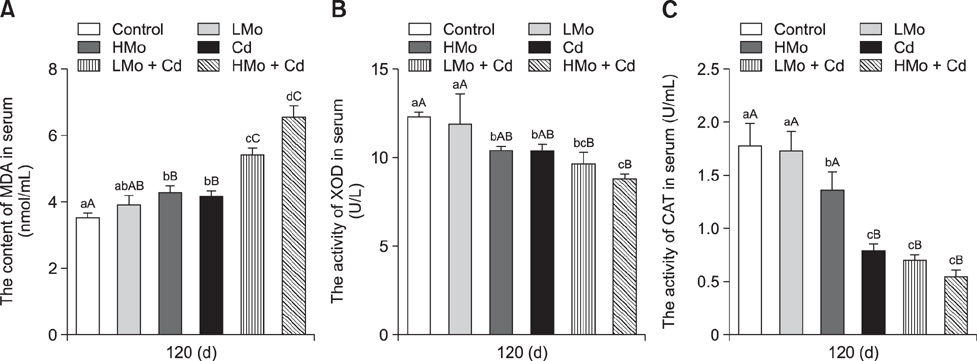
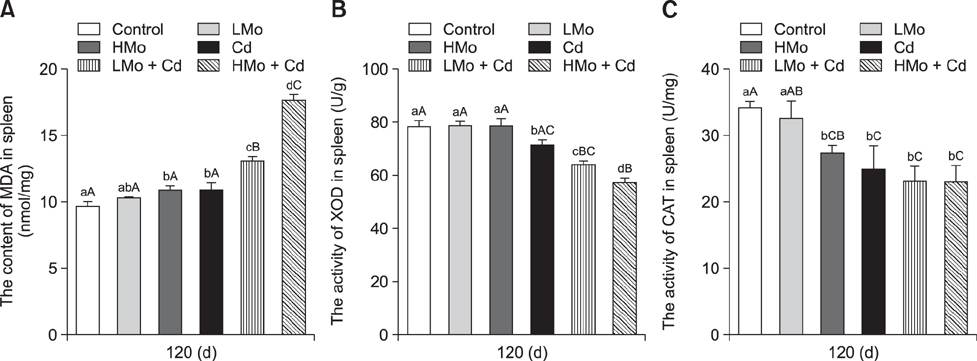
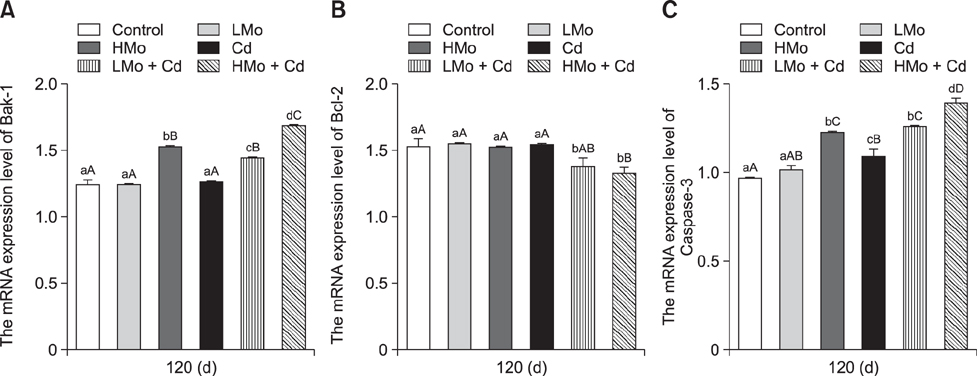
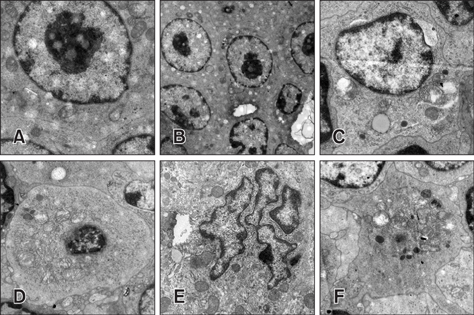
- To investigate the effects of molybdenum (Mo) and/or cadmium (Cd) on antioxidant function and the apoptosis-related genes in duck spleens. Sixty healthy 11-day-old ducks were randomly divided into six groups of 10 ducks (control, low Mo group, high Mo, Cd, low Mo + Cd, and high Mo + Cd groups). All were fed a basal diet containing low or high dietary doses of Mo and/or Cd. Relative spleen weight, antioxidant indices, apoptosis-related gene mRNA expression levels, and ultrastructural changes were evaluated after 120 days. The results showed that the relative spleen weight decreased significantly in the high Mo + Cd treatment group which compared with control group. Malondialdehyde levels increased and xanthine oxidase and catalase activities decreased in the Mo and/or Cd groups compared with levels in the control group. Bak-1 and Caspase-3 expressions were upregulated in the high Mo + Cd group, while Bcl-2 was downregulated. In addition, mitochondrial crest fracture, swelling, vacuolation, deformed nuclei, and karyopyknosis in both Mo + Cd treated groups were more severe than in the other groups. The results suggest that Mo and/or Cd can induce oxidative stress and apoptosis of spleen via effects on the mitochondrial intrinsic pathway. Moreover, the results indicate the two elements have a possible synergistic relationship.
Keyword
MeSH Terms
-
Animals
Antioxidants/*metabolism/physiology
Apoptosis/*drug effects
Cadmium/*toxicity
Catalase/metabolism
Dose-Response Relationship, Drug
Ducks/metabolism
Gene Expression/drug effects
Malondialdehyde/metabolism
Microscopy, Electron, Transmission/veterinary
Molybdenum/*toxicity
Organ Size/drug effects
Real-Time Polymerase Chain Reaction/veterinary
Spleen/*drug effects/ultrastructure
Xanthine Oxidase/metabolism
Antioxidants
Cadmium
Malondialdehyde
Molybdenum
Catalase
Xanthine Oxidase
Figure
Reference
-
1. Barceloux DG. Molybdenum. J Toxicol Clin Toxicol. 1999; 37:231–237.
Article2. Bekheet SHM, Awadalla EA, Salman MM, Hassan MK. Bradykinin potentiating factor isolated from Buthus occitanus has a protective effect against cadmium-induced rat liver and kidney damage. Tissue Cell. 2011; 43:337–343.
Article3. Bersényi A, Berta E, Kádár I, Glávits R, Szilágyi M, Fekete SG. Effects of high dietary molybdenum in rabbits. Acta Vet Hung. 2008; 56:41–55.
Article4. Cannino G, Ferruggia E, Luparello C, Rinaldi AM. Cadmium and mitochondria. Mitochondrion. 2009; 9:377–384.
Article5. Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K. Cadmium stress: an oxidative challenge. Biometals. 2010; 23:927–940.
Article6. Davies TD, Pickard J, Hall KJ. Acute molybdenum toxicity to rainbow trout and other fish. J Environ Eng Sci. 2005; 4:481–485.
Article7. Dwivedi VK, Bhatanagar A, Chaudhary M. Protective role of ceftriaxone plus sulbactam with VRP1034 on oxidative stress, hematological and enzymatic parameters in cadmium toxicity induced rat model. Interdiscip Toxicol. 2012; 5:192–200.
Article8. Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai). 2005; 37:719–727.
Article9. Fu J, Liu CP, Zhang ZW, Xing MW, Xu SW. Influence of inflammatory pathway markers on oxidative stress induced by cold stress in intestine of quails. Res Vet Sci. 2013; 95:495–501.
Article10. Gao D, Xu Z, Qiao P, Liu S, Zhang L, He P, Zhang X, Wang Y, Min W. Cadmium induces liver cell apoptosis through caspase-3A activation in purse red common carp (Cyprinus carpio). PLoS One. 2013; 8:e83423.11. Gobe G, Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicol Lett. 2010; 198:49–55.
Article12. Gu X, Ali T, Chen R, Hu G, Zhuang Y, Luo J, Cao H, Han B. In vivo studies of molybdenum-induced apoptosis in kidney cells of caprine. Biol Trace Elem Res. 2015; 165:51–58.
Article13. Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998; 19:529–535.14. Kiersztan A, Winiarska K, Drozak J, Przedlacka M, Wegrzynowicz M, Fraczyk T, Bryla J. Differential effects of vanadium, tungsten and molybdenum on inhibition of glucose formation in renal tubules and hepatocytes of control and diabetic rabbits: beneficial action of melatonin and N-acetylcysteine. Mol Cell Biochem. 2004; 261:9–21.
Article15. Larsen BD, Rampalli S, Burns LE, Brunette S, Dilworth FJ, Megeney LA. Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks. Proc Natl Acad Sci U S A. 2010; 107:4230–4235.
Article16. Li JL, Jiang CY, Li S, Xu SW. Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicol Environ Saf. 2013; 96:103–109.
Article17. Li JL, Li HX, Li S, Tang ZX, Xu SW, Wang XL. Oxidative stress-mediated cytotoxicity of cadmium in chicken splenic lymphocytes. Polish J Environ Stud. 2010; 19:947–956.
Article18. Liu J, Yao Y, Ding H, Chen R. Oxymatrine triggers apoptosis by regulating Bcl-2 family proteins and activating caspase-3/caspase-9 pathway in human leukemia HL-60 cells. Tumour Biol. 2014; 35:5409–5415.
Article19. Liu S, Xu FP, Yang ZJ, Li M, Min YH, Li S. Cadmium-induced injury and the ameliorative effects of selenium on chicken splenic lymphocytes: mechanisms of oxidative stress and apoptosis. Biol Trace Elem Res. 2014; 160:340–351.
Article20. Liu X, Li Z, Tie F, Liu N, Zhang Z, Xu S. Effects of manganese-toxicity on immune-related organs of cocks. Chemosphere. 2013; 90:2085–2100.
Article21. Liu X, Zhang L, Guan H, Zhang Z, Xu S. Effects of oxidative stress on apoptosis in manganese-induced testicular toxicity in cocks. Food Chem Toxicol. 2013; 60:168–176.
Article22. Llambi F, Moldoveanu T, Tait SWG, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011; 44:517–531.
Article23. Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005; 58:730–735.
Article24. Nemmiche S, Chabane-Sari D, Kadri M, Guiraud P. Cadmium chloride-induced oxidative stress and DNA damage in the human Jurkat T cell line is not linked to intracellular trace elements depletion. Toxicology In Vitro. 2011; 25:191–198.
Article25. Pathak N, Khandelwal S. Role of oxidative stress and apoptosis in cadmium induced thymic atrophy and splenomegaly in mice. Toxicol Lett. 2007; 169:95–108.
Article26. Prozialeck WC, Edwards JR. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J Pharmacol Exp Ther. 2012; 343:2–12.
Article27. Sagor MAT, Tabassum N, Potol MA, Alam MA. Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxid Med Cell Longev. 2015; 2015:478039.
Article28. Sahin E, Gümüşlü S. Cold-stress-induced modulation of antioxidant defence: role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int J Biometeorol. 2004; 48:165–171.
Article29. Shao JJ, Yao HD, Zhang ZW, Li S, Xu SW. The disruption of mitochondrial metabolism and ion homeostasis in chicken hearts exposed to manganese. Toxicol Lett. 2012; 214:99–108.
Article30. Siddiqui MA, Saquib Q, Ahamed M, Farshori NN, Ahmad J, Wahab R, Khan ST, Alhadlaq HA, Musarrat J, Al-Khedhairy AA, Pant AB. Molybdenum nanoparticles-induced cytotoxicity, oxidative stress, G2/M arrest, and DNA damage in mouse skin fibroblast cells (L929). Colloids Surf B Biointerfaces. 2015; 125:73–81.
Article31. Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015; 89:289–317.
Article32. Szegezdi E, MacDonald DC, Ni Chonghaile T, Gupta S, Samali A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009; 296:C941–C953.
Article33. Tayarani-Najaran Z, Mousavi SH, Vahdati-Mashhadian N, Emami SA, Parsaee H. Scutellaria litwinowii induces apoptosis through both extrinsic and intrinsic apoptotic pathways in human promyelocytic leukemia cells. Nutr Cancer. 2012; 64:80–88.
Article34. Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998; 3:697–707.
Article35. Wang J, Zhu H, Liu X, Liu Z. Oxidative stress and Ca2+ signals involved on cadmium-induced apoptosis in rat hepatocyte. Biol Trace Elem Res. 2014; 161:180–189.
Article36. Wang JY, Luo ZG. Non-apoptotic role of caspase-3 in synapse refinement. Neurosci Bull. 2014; 30:667–670.
Article37. Wang Y, Fang J, Leonard SS, Rao KM. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004; 36:1434–1443.
Article38. Xia B, Cao H, Luo J, Liu P, Guo X, Hu G, Zhang C. The co-induced effects of molybdenum and cadmium on antioxidants and heat shock proteins in duck kidneys. Biol Trace Elem Res. 2015; 168:261–268.
Article39. Xiao J, Cui HM, Yang F, Peng X, Cui Y. Effect of dietary high molybdenum on the cell cycle and apoptosis of kidney in broilers. Biol Trace Elem Res. 2011; 142:523–531.
Article40. Yang F, Cui H, Xiao J, Peng X, Deng J, Zuo Z. Increased apoptotic lymphocyte population in the spleen of young chickens fed on diets high in molybdenum. Biol Trace Elem Res. 2011; 140:308–316.
Article41. Zhang M, He Z, Wen L, Wu J, Yuan L, Lu Y, Guo C, Zhu L, Deng S, Yuan H. Cadmium suppresses the proliferation of piglet Sertoli cells and causes their DNA damage, cell apoptosis and aberrant ultrastructure. Reprod Biol Endocrinol. 2010; 8:97.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats
- Blood and urine cadmium levels in non-exposed Korean to cadmium
- Antioxidant activities and nutritional characteristics of smoked duck marinated in natural curing agent
- Effect of Cadmium Chloride on the Cardiac Muscle Ultrastructure in Rats
- A Study on Antibody Producing by Intoxication of Cadmium Chloride or Lead Acetate in Rat