J Vet Sci.
2017 Sep;18(3):377-386. 10.4142/jvs.2017.18.3.377.
Effect of canine mesenchymal stromal cells overexpressing heme oxygenase-1 in spinal cord injury
- Affiliations
-
- 1BK21 PLUS Program for Creative Veterinary Science Research, Research Institute for Veterinary Science and College of Veterinary Medicine, Seoul National University, Seoul 08872, Korea. ohkweon@snu.ac.kr
- KMID: 2412456
- DOI: http://doi.org/10.4142/jvs.2017.18.3.377
Abstract
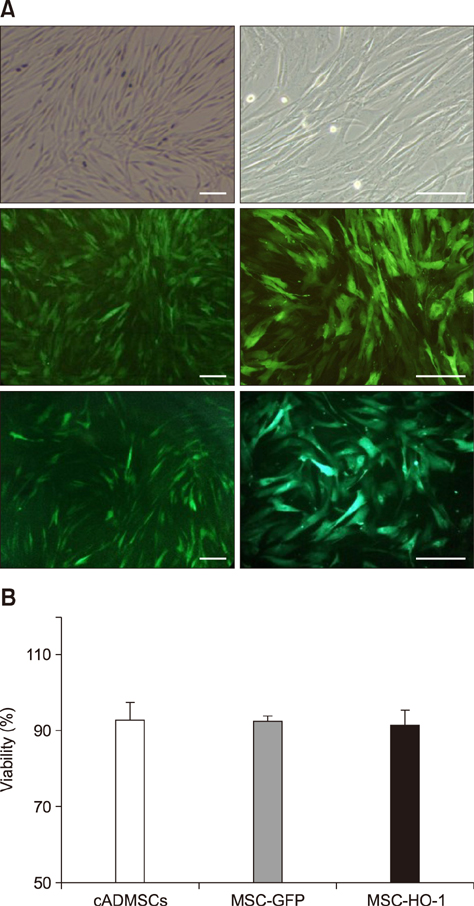
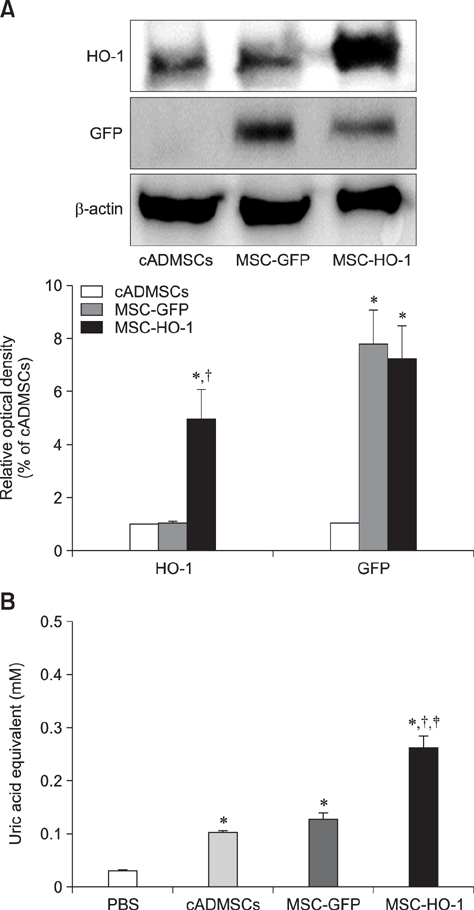
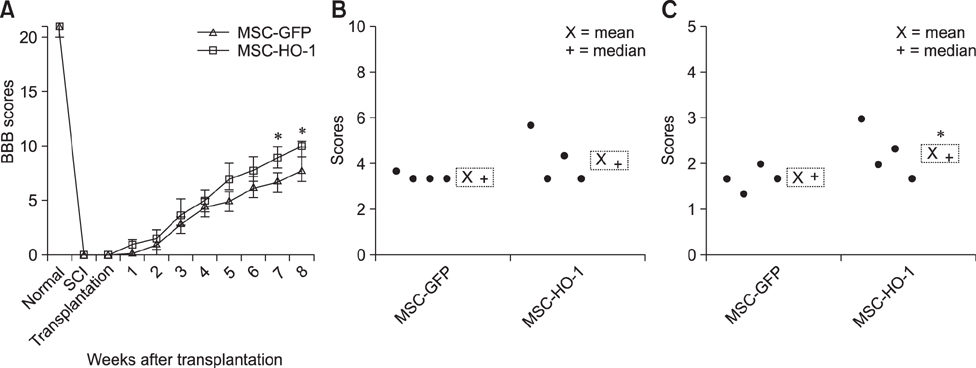
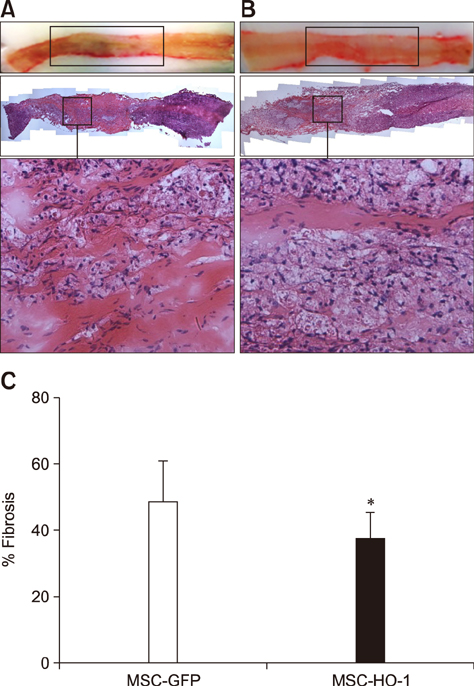
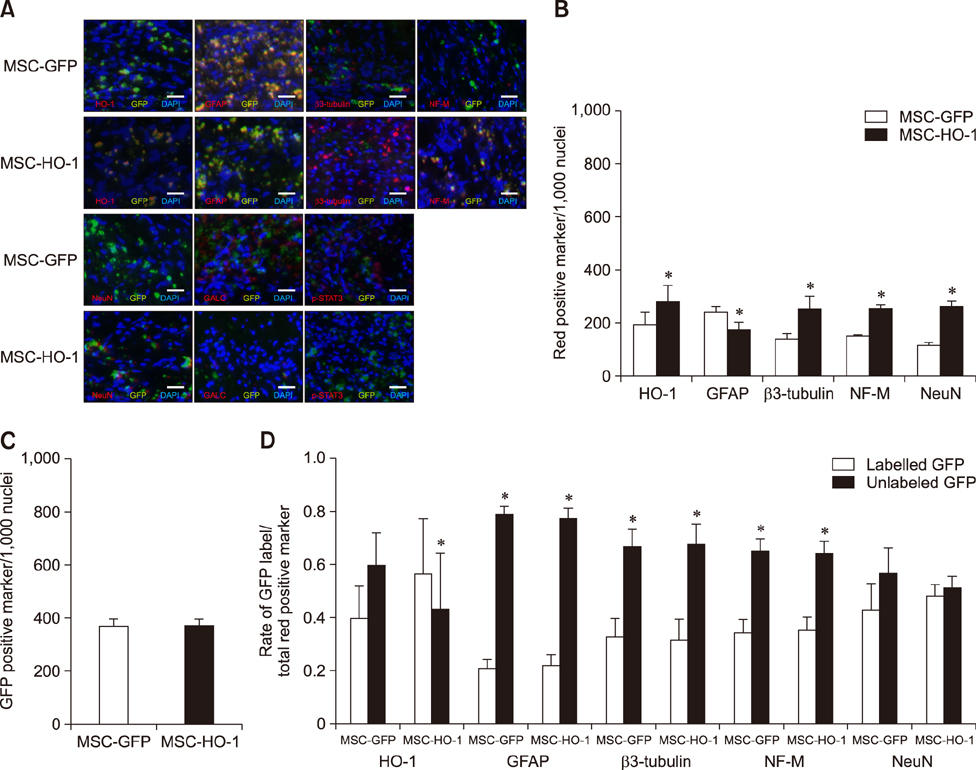
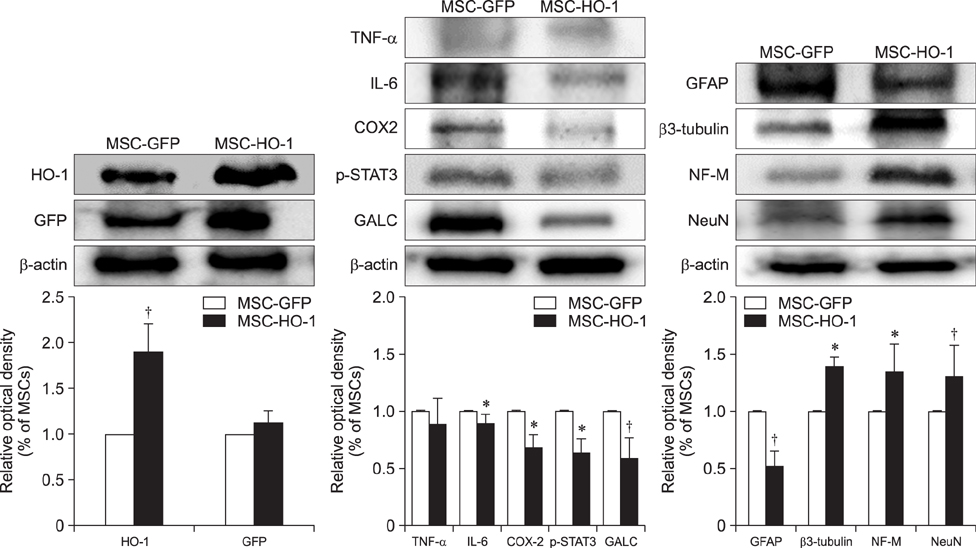
- Heme oxygenase-1 (HO-1) is a stress-responsive enzyme that modulates the immune response and oxidative stress associated with spinal cord injury (SCI). This study aimed to investigate neuronal regeneration via transplantation of mesenchymal stromal cells (MSCs) overexpressing HO-1. Canine MSCs overexpressing HO-1 were generated by using a lentivirus packaging protocol. Eight beagle dogs with experimentally-induced SCI were divided into GFP-labeled MSC (MSC-GFP) and HO-1-overexpressing MSC (MSC-HO-1) groups. MSCs (1 × 10ⷠcells) were transplanted at 1 week after SCI. Spinal cords were harvested 8 weeks after transplantation, after which histopathological, immunofluorescence, and western blot analyses were performed. The MSC-HO-1 group showed significantly improved functional recovery at 7 weeks after transplantation. Histopathological results showed fibrotic changes and microglial cell infiltration were significantly decreased in the MSC-HO-1 group. Immunohistochemical (IHC) results showed significantly increased expression levels of HO-1 and neuronal markers in the MSC-HO-1 group. Western blot results showed significantly decreased expression of tumor necrosis factor-alpha, interleukin-6, cycloogygenase 2, phosphorylated-signal transducer and activator of transcription 3, and galactosylceramidase in the MSC-HO-1 group, while expression levels of glial fibrillary acidic protein, β3-tubulin, neurofilament medium, and neuronal nuclear antigen were similar to those observed in IHC results. Our results demonstrate that functional recovery after SCI can be promoted to a greater extent by transplantation of HO-1-overexpressing MSCs than by normal MSCs.
MeSH Terms
Figure
Reference
-
1. Andrews EM, Tsai SY, Johnson SC, Farrer JR, Wagner JP, Kopen GC, Kartje GL. Human adult bone marrow-derived somatic cell therapy results in functional recovery and axonal plasticity following stroke in the rat. Exp Neurol. 2008; 211:588–592.
Article2. Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta. 2012; 1822:675–684.
Article3. Barros Filho TE, Molina AE. Analysis of the sensitivity and reproducibility of the Basso, Beattie, Bresnahan (BBB) scale in Wistar rats. Clinics (Sao Paulo). 2008; 63:103–108.
Article4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.
Article5. Cho SR, Kim YR, Kang HS, Yim SH, Park CI, Min YH, Lee BH, Shin JC, Lim JB. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone barrow in a rat model of spinal cord injury. Cell Transplant. 2009; 18:1359–1368.
Article6. Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000; 7:574–585.
Article7. Emgård M, Hallin U, Karlsson J, Bahr BA, Brundin P, Blomgren K. Both apoptosis and necrosis occur early after intracerebral grafting of ventral mesencephalic tissue: a role for protease activation. J Neurochem. 2003; 86:1223–1232.
Article8. Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008; 28:7231–7243.
Article9. Kanno H, Ozawa H, Dohi Y, Sekiguchi A, Igarashi K, Itoi E. Genetic ablation of transcription repressor Bach1 reduces neural tissue damage and improves locomotor function after spinal cord injury in mice. J Neurotrauma. 2009; 26:31–39.
Article10. Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005; 25:4694–4705.
Article11. Kim M, Kim Y, Lee S, Kuk M, Kim AY, Kim W, Kweon OK. Comparison of viability and antioxidant capacity between canine adipose-derived mesenchymal stem cells and heme oxygenase-1-overexpressed cells after freeze-thawing. J Vet Med Sci. 2016; 78:619–625.
Article12. Kim Y, Jo SH, Kim WH, Kweon OK. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res Ther. 2015; 6:229.
Article13. Kuk M, Kim Y, Lee SH, Kim WH, Kweon OK. Osteogenic ability of canine adipose-derived mesenchymal stromal cell sheets in relation to culture time. Cell Transplant. 2016; 25:1415–1422.
Article14. Lee SH, Kim Y, Rhew D, Kuk M, Kim M, Kim WH, Kweon OK. Effect of the combination of mesenchymal stromal cells and chondroitinase ABC on chronic spinal cord injury. Cytotherapy. 2015; 17:1374–1383.
Article15. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007; 8:275–282.
Article16. Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol. 2007; 203:8–21.
Article17. Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005; 191:344–360.
Article18. Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013; 31:701–713.
Article19. Öllinger R, Pratschke J. Role of heme oxygenase-1 in transplantation. Transpl Int. 2010; 23:1071–1081.
Article20. Origassa CS, Câmara NO. Cytoprotective role of heme oxygenase-1 and heme degradation derived end products in liver injury. World J Hepatol. 2013; 5:541–549.
Article21. Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, Kocsis JD. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010; 1343:226–235.
Article22. Park SS, Byeon YE, Ryu HH, Kang BJ, Kim Y, Kim WH, Kang KS, Han HJ, Kweon OK. Comparison of canine umbilical cord blood-derived mesenchymal stem cell transplantation times: involvement of astrogliosis, inflammation, intracellular actin cytoskeleton pathways, and neurotrophin-3. Cell Transplant. 2011; 20:1867–1880.
Article23. Park SS, Lee YJ, Lee SH, Lee D, Choi K, Kim WH, Kweon OK, Han HJ. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal stem cells. Cytotherapy. 2012; 14:584–597.
Article24. Rabinowitz RS, Eck JC, Harper CM Jr, Larson DR, Jimenez MA, Parisi JE, Friedman JA, Yaszemski MJ, Currier BL. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine (Phila Pa 1976). 2008; 33:2260–2268.
Article25. Ryu HH, Byeon YE, Park SS, Kang BJ, Seo MS, Park SB, Kim WH, Kang KS, Kweon OK. Immunohistomorphometric analysis of transplanted umbilical cord blood-derived mesenchymal stem cells and rhe resulting anti-inflammatory effects on nerve regeneration of injured canine spinal cord. Tissue Eng Regen Med. 2011; 8:173–182.26. Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YW, Kim WH, Kang KS, Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009; 10:273–284.
Article27. Snyder EY, Teng YD. Stem cells and spinal cord repair. N Engl J Med. 2012; 366:1940–1942.
Article28. Soares MP, Marguti I, Cunha A, Larsen R. Immunoregulatory effects of HO-1: how does it work? Curr Opin Pharmacol. 2009; 9:482–489.
Article29. Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005; 46:1339–1350.
Article30. Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006; 26:9713–9721.
Article31. Torres-Espín A, Redondo-Castro E, Hernandez J, Navarro X. Immunosuppression of allogenic mesenchymal stem cells transplantation after spinal cord injury improves graft survival and beneficial outcomes. J Neurotrauma. 2015; 32:367–380.
Article32. Uemura M, Refaat MM, Shinoyama M, Hayashi H, Hashimoto N, Takahashi J. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010; 88:542–551.
Article33. Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma. 2004; 21:1017–1030.
Article34. Yeom HJ, Koo OJ, Yang J, Cho B, Hwang JI, Park SJ, Hurh S, Kim H, Lee EM, Ro H, Kang JT, Kim SJ, Won JK, O'Connell PJ, Kim H, Surh CD, Lee BC, Ahn C. Generation and characterization of human heme oxygenase-1 transgenic pigs. PLoS One. 2012; 7:e46646.
Article35. Zhang LF, Qi J, Zuo G, Jia P, Shen X, Shao J, Kang H, Yang H, Deng L. Osteoblast-secreted factors promote proliferation and osteogenic differentiation of bone marrow stromal cells via VEGF/heme-oxygenase-1 pathway. PLoS One. 2014; 9:e99946.
Article36. Zhang M, Zhang BH, Chen L, An W. Overexpression of heme oxygenase-1 protects smooth muscle cells against oxidative injury and inhibits cell proliferation. Cell Res. 2002; 12:123–132.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lithium ameliorates rat spinal cord injury by suppressing glycogen synthase kinase-3β and activating heme oxygenase-1
- Preclinical Efficacy and Mechanisms of Mesenchymal Stem Cells in Animal Models of Autoimmune Diseases
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Effect of the Heme Oxygenase Inhibitor on the Hypoxic Ischemic Brain Injury in the Neonatal Rat
- Clinical Response of 277 Patients with Spinal Cord Injury to Stem Cell Therapy in Iraq