Yonsei Med J.
2018 Jun;59(4):563-565. 10.3349/ymj.2018.59.4.563.
Increased Uptake of AV-1451 in a Subacute Infarction Lesion
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. evekhj@gmail.com
- 2Neuroscience Center, Samsung Medical Center, Seoul, Korea.
- 3Department of Neurology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Nuclear Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Division of RI-Convergence Research, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 6Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul, Korea.
- 7Department of Clinical Research Design and Evaluation, SAIHST, Sungkyunkwan University, Seoul, Korea.
- KMID: 2410912
- DOI: http://doi.org/10.3349/ymj.2018.59.4.563
Abstract
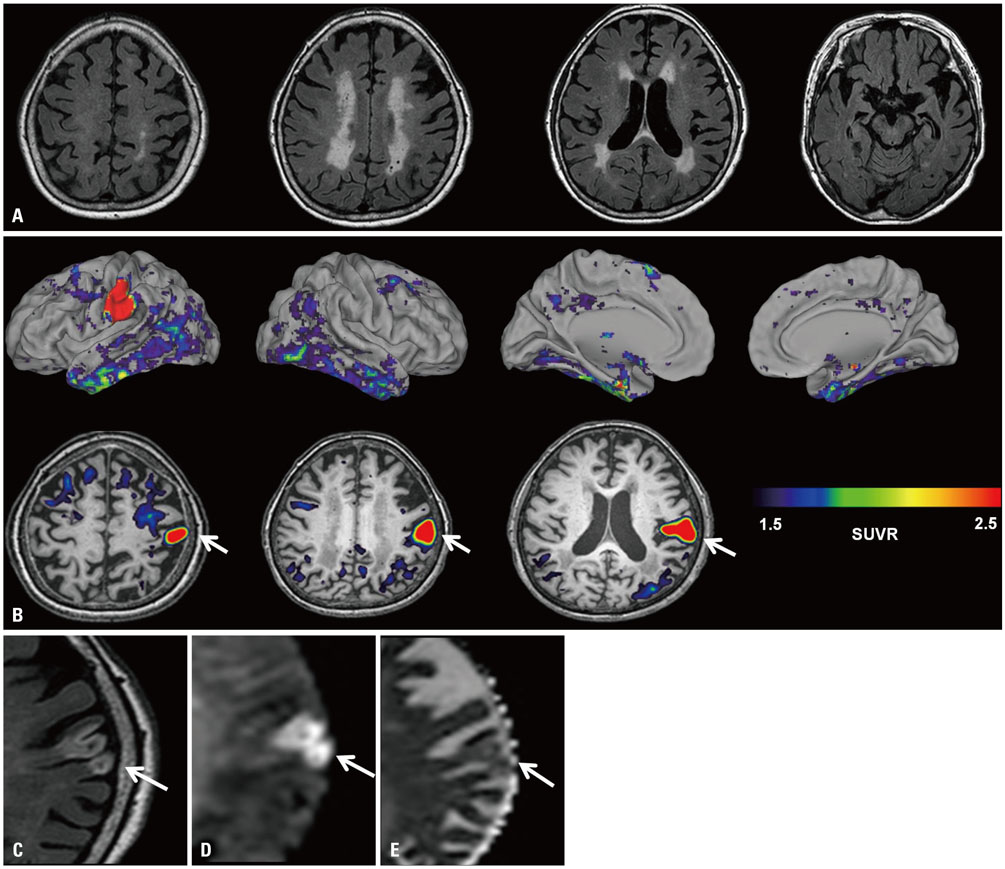
- ¹â¸F-AV-1451 is a tau PET ligand that has high affinity for paired helical filament tau. However, various off-target bindings unrelated to tau have also been reported. Herein, we report a case of 83-year-old woman, who showed abnormal uptake of AV-1451 that was shown to be subacute infarction. Clinicians should recognize that increased uptake of AV-1451 may be related to stroke.
Figure
Reference
-
1. Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016; 80:247–258.
Article2. Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia C, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis. 2014; 38:171–184.
Article3. Lockhart SN, Ayakta N, Winer JR, La Joie R, Rabinovici GD, Jagust WJ. Elevated 18F-AV-1451 PET tracer uptake detected in incidental imaging findings. Neurology. 2017; 88:1095–1097.
Article4. Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016; 4:58.
Article5. Marquié M, Verwer EE, Meltzer AC, Kim SJW, Agüero C, Gonzalez J, et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson's case. Acta Neuropathol Commun. 2017; 5:75.
Article6. Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010; 25:1071–1076.
Article7. Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016; 139(Pt 5):1551–1567.
Article8. Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016; 79:110–119.
Article9. Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain. 2015; 138(Pt 5):1370–1381.
Article10. Wen Y, Yang S, Liu R, Simpkins JW. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain. 2004; 1022:30–38.
Article11. Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Muñoz Maniega S, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009; 65:194–202.
Article12. Petito CK. Early and late mechanisms of increased vascular permeability following experimental cerebral infarction. J Neuropathol Exp Neurol. 1979; 38:222–234.
Article13. Ly JV, Rowe CC, Villemagne VL, Zavala JA, Ma H, Sahathevan R, et al. Subacute ischemic stroke is associated with focal 11C PiB positron emission tomography retention but not with global neocortical Aβ deposition. Stroke. 2012; 43:1341–1346.
Article14. Sahathevan R, Linden T, Villemagne VL, Churilov L, Ly JV, Rowe C, et al. Positron emission tomographic imaging in stroke: crosssectional and follow-up assessment of amyloid in ischemic stroke. Stroke. 2016; 47:113–119.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Differences of the Magnetic Resonance Spectroscopy Findings Between a Subacute Infarction and a Malignant Glioma with a Similar Mass-Like Enhancing Lesion
- AV Conduction Disturbances Associated with Acute Myocardial Infarction
- An experimental study on radionuclide imaging of bowel infarction using (99m)Tc-pyrophosphate
- Comparison of Tc-99m-HMPAO SPECT and MRI in cerebral infarction
- Three Cases of Renal Infarction